Surgery for Valvular Endocarditis
Gosta B. Pettersson
Syed T. Hussain
Introduction
Infective endocarditis (IE) is one of the most severe and potentially devastating complications of heart valve disease, be it native valve (NVE), prosthetic valve (PVE), or infection on another cardiac device. As a consequence of more cardiac interventions, an increasingly elderly population with degenerative valvular heart disease, and a rise in staphylococcal infections, the number of patients at higher risk of endocarditis is growing. Despite advances in surgical techniques, operations for IE remain associated with the highest mortality of any valve disease. The clinical scenarios presented by patients with IE are often very complex and require prompt diagnosis and institution of antibiotic treatment and decision making involving high-risk surgery. We believe that a team approach and thoughtful decisions regarding indications for and timing of surgery and execution of the operation with radical debridement and reconstruction of the heart will improve chances of survival and outcomes for patients with IE.
Residual Controversies Regarding the Value of Surgery for Treatment of Endocarditis
All patients diagnosed with IE are treated with antibiotics, and when IE is cured, it is cured by antibiotics and the patients’ own defense systems. This is true whether the patient undergoes surgery or not. The question is, does surgery improve the chances of cure?
Before the era of antibiotics, IE was a uniformly fatal disease, and the concepts acute, subacute, and chronic endocarditis only referred to how long the patient would survive, in essence to the aggressiveness of the causing organism. We have recently described the progression and the stages of the disease. The organisms adhere to areas with endocardial damage, where they form colonies (vegetations); multiply and produce enzymes killing cells and disintegrating tissue, cusps, and leaflets; and make invasion
of extravascular structures possible. Antibiotics to which the organisms are sensitive may halt further destruction, but they will not restore the integrity of damaged tissue. In patients with prosthetic valve or other device infections or invasive disease, antibiotics are less likely to reach the organisms in adequate concentrations. Different organisms have different mechanisms by which they protect themselves from antibiotics (e.g., biofilm formation) and produce different enzymes and other toxic substances modulating their virulence, with Staphylococcus aureus being particularly virulent. Cure of PVE or other device infection is even more unlikely without surgical removal of the infected foreign body.
of extravascular structures possible. Antibiotics to which the organisms are sensitive may halt further destruction, but they will not restore the integrity of damaged tissue. In patients with prosthetic valve or other device infections or invasive disease, antibiotics are less likely to reach the organisms in adequate concentrations. Different organisms have different mechanisms by which they protect themselves from antibiotics (e.g., biofilm formation) and produce different enzymes and other toxic substances modulating their virulence, with Staphylococcus aureus being particularly virulent. Cure of PVE or other device infection is even more unlikely without surgical removal of the infected foreign body.
Surgery removes vegetations, infected necrotic tissue, and foreign material and also drains infected areas, thereby improving the access of antibiotics to cure the infection. In addition, valve repair or replacement restores valve function and cardiac integrity. Still, cure is the result of antibiotics! Most experienced groups, including ours, are convinced that surgery is beneficial and are becoming increasingly aggressive about advocating earlier surgery rather than delaying it. This evolution is based on a growing clinical experience with surgery demonstrating better outcomes. Studies that still question the value of surgery use methodology that takes into account survival bias—the self-selection of patients surviving long enough to be considered for surgery—by looking at entire populations with IE to study whether or not addition of surgery improves outcomes. As surgeons, we are limited to studying patients who are still alive when we first see them; thus, survival bias will always be an issue in these studies. However, with the present improved outcomes, randomized studies will have to be very well planned and address a specific question in well-defined patient groups. Kang and coworkers recently published one randomized study specifically targeting timing of surgery and were able to provide evidence supporting earlier surgery rather than waiting for heart failure. The indications we present in this chapter represent our current position.
In general, our recommendation is that once a surgical indication is present, surgery should not be delayed. Outcomes are not related to the duration of antibiotic therapy before surgery, and there seems to be no penalty for operating while IE is still active. If the organism, however, happens to be insensitive to the antibiotics given at the time of surgery, the risk of noncure and recurrent infection is increased.
Indications for surgery in patients with IE are presented in Table 43.1. The only generally accepted indications for surgery in patients with active IE are congestive heart failure related to a visible hemodynamic issue like severe valve regurgitation, stenosis, or fistula and PVE with evidence of anulus involvement, particularly if the causative organism is S. aureus. Early surgery means operating for any of these conditions before heart failure has developed. Approximately half of the patients with IE develop severe complications that sooner or later require an operation.
TABLE 43.1 Indications for Surgery in Patients With IE | |
|---|---|
|
In patients with NVE, congestive heart failure is the most frequent and severe complication of IE and is a class I indication for surgery. We consider presence of perivalvular extension and invasive disease or “difficult to treat” organisms causing uncontrolled infection indications for early intervention in those with NVE, unless the patient is inoperable from severe comorbidities or brain damage. Prevention of embolism in patients with large mobile vegetations is a more controversial indication. The risk of embolism is highest in the first 2 weeks of antibiotic therapy and is related to the location, size, and mobility of the vegetation. Although all these qualities of vegetations influence the indication for surgery, previous embolism, type of organism, and duration of antibiotic therapy are also considered.
Patients with complicated PVE, staphylococcal PVE, and early PVE require aggressive management that includes early surgery. For patients with uncomplicated, non-staphylococcal, and late PVE, treatment with antibiotics alone may be worth trying, but often the infection will recur within a few months.
All patients scheduled for surgery for IE should have complete neurologic evaluation and brain imaging, either by computed tomography or magnetic resonance imaging. We also recommend that patients with intracranial bleeding undergo cerebral angiography to exclude mycotic aneurysms, although the yield will be low. A mycotic aneurysm deemed to be high risk for rupture should be clipped or embolized before the heart operation. Delay of the heart operation is usually advised in patients with more serious neurologic complications. We do not operate on unconscious patients or those unable to follow simple commands until neurologic improvement has been demonstrated. Patients with hemorrhagic strokes should have heart surgery delayed at least 2 weeks, preferably 3 to 4 weeks, to reduce the risk of further bleeding during the operation. For those with nonhemorrhagic embolic strokes, the main concerns are worsening the neurologic damage and hemorrhagic conversion of the infarct during the operation. Although surgery is relatively safe, if the patient is hemodynamically stable and risk of further embolism is deemed low, the chances of worsening the neurologic injury in the operating room diminish week by week during the first 3 weeks after an ischemic event; we routinely try to delay surgery in these cases for 1 to 2 weeks. The risk of worsening the neurologic injury must be weighed against the patient’s hemodynamic situation and risk of additional emboli while waiting.
Patient management plus indications for and timing of surgery are discussed by a multispecialty team dedicated to managing patients with IE. The management team should include an imaging cardiologist, an infectious disease specialist, and a cardiac surgeon. A neurologist and sometimes a neurosurgeon become involved when neurologic complications are present, and a nephrologist may be needed to manage renal failure. A psychiatrist and a social worker help manage drug addicts. Other specialists are consulted as required for a particular patient.
A bacteriologic diagnosis should be secured by repeated blood cultures before starting antibiotics. Immediately after, an antibiotic regimen covering all suspected organisms should be promptly initiated to halt further bacterial destruction of valve cusps and leaflets and paravalvular invasion. Once there is an indication for surgery and sensitivity of the organism to the given antibiotics has been confirmed, there is no additional benefit to delaying surgery to allow a longer period of preoperative antibiotic treatment.
Preoperative imaging should include echocardiograms, computed tomography scanning or magnetic resonance imaging of the chest, and cardiac catheterization. Safe sternal reentry in cases of reoperation requires knowledge about structures at risk of injury. When arterial structures such as the ascending aorta, a pseudoaneurysm, or an important graft are in direct contact with the sternum, consideration should be given to peripheral cannulation (preferably via the axillary artery) and institution of cardiopulmonary bypass before sternotomy is completed.
General Principles
Objectives of surgery for IE are to prevent additional embolic events, debride and remove all infected tissues and foreign materials, and restore functional valves and cardiac integrity. If the infection is limited to the valve cusps or leaflets (referred to as “simple” IE) of a native valve, replacement with either a biologic or mechanical valve prosthesis according to the same principles as for noninfected valves is acceptable.
Advanced pathologies (extravascular invasion beyond the cusps or leaflets) require radical debridement and reconstruction. To reconstruct the aortic root, an aortic allograft is our preferred choice, with minimal use of additional foreign material. Mitral valves should be repaired whenever possible. Both radical debridement and subsequent reconstruction are easier and better achieved in patients with advanced aortic IE, as compared with mitral valve IE, because of anatomical and surgical obstacles related to the mitral valve. This will be discussed in more detail later.
Techniques
Intraoperative transesophageal echocardiography is mandatory!
Operations for IE are best performed through a full median sternotomy. For mitral PVE, an alternative fourth interspace right anterolateral thoracotomy approach can be considered, but this allows only limited access and denies an opportunity to evaluate the aortic valve; thus, it is acceptable only in patients with isolated, noninvasive mitral IE.
Because patients who require surgery for IE are generally very sick and the procedure is often long and complex, perfect myocardial protection is critical. This is achieved using initial induction with antegrade and retrograde blood cardioplegia, and repeat retrograde cardioplegia every 15 to 20 minutes. To guarantee delivery of retrograde cardioplegia, we open the right atrium and cannulate the coronary sinus directly through a purse string around its orifice. In patients with large mobile vegetations, it is preferable to minimize manipulations before arresting the heart.
Aortic Valve Endocarditis
The aortic valve is explored through a transverse aortotomy 1 cm or more above the right coronary artery. For noninvasive NVE with limited localized infection and preserved contours of the cusps, an attempt can be made to repair and preserve the valve. The vegetations are removed and the resulting defect in the cusp repaired with an autologous pericardial patch. When preservation of the cusps is not possible, the reality in most cases, complete removal of the cusps and implantation of a biologic or mechanical valve is required. Choice of prosthetic valve follows normal principles of valve surgery, based on age, comorbidities, life expectancy, and expected compliance with anticoagulation.
In every IE case, we look carefully for invasion because a small entry may hide an extensive extra-aortic spread of the infection (Fig. 43.1A,B). If the patient has heart block of any degree, the infection is likely to be close to the atrioventricular (AV) node and bundle, and the right atrium must be opened for inspection (Fig. 43.2A,B). For optimal surgical treatment of PVE and advanced aortic valve IE, the pathology and anatomy of the left ventricular outflow tract (LVOT) must be fully understood. Surgery for aortic root abscess and/or cardiac fistulas requires complete debridement of all infected tissues and foreign materials (prosthesis, pledgets, and sutures), and the debrided area should look clean and free from infected and necrotic tissue (Fig. 43.3). While striving for complete debridement, caution must be taken not to lose track of the anatomy and overdo the debridement by cutting into coronaries and sacrificing live outflow tract muscle.
An aortic allograft is our preferred choice for reconstructing the aortic root and LVOT, but this is no substitute for radical removal of all infected tissues: Even allografts
are not immune to reinfection. The LVOT is almost always preserved to allow direct anastomosis of the allograft (Fig. 43.3). The intervalvular fibrosa (IVF) corresponding to the base of the anterior mitral valve leaflet and the two trigones on either side are the main landmarks guiding the reconstruction and indicate the level of the proximal suture line. The LVOT is sized with Hegar dilators, and an allograft with an internal diameter 2 to 3 mm less than the diameter of the anulus is chosen. Correct sizing of the allograft is important. When only a smaller allograft is available, the anulus is reduced and supported by placing two 2-0 Gortex sutures around the anulus and tying them over a Hegar
dilator of chosen diameter. The coronary buttons should be well mobilized and generous in size to facilitate future reoperation.
are not immune to reinfection. The LVOT is almost always preserved to allow direct anastomosis of the allograft (Fig. 43.3). The intervalvular fibrosa (IVF) corresponding to the base of the anterior mitral valve leaflet and the two trigones on either side are the main landmarks guiding the reconstruction and indicate the level of the proximal suture line. The LVOT is sized with Hegar dilators, and an allograft with an internal diameter 2 to 3 mm less than the diameter of the anulus is chosen. Correct sizing of the allograft is important. When only a smaller allograft is available, the anulus is reduced and supported by placing two 2-0 Gortex sutures around the anulus and tying them over a Hegar
dilator of chosen diameter. The coronary buttons should be well mobilized and generous in size to facilitate future reoperation.
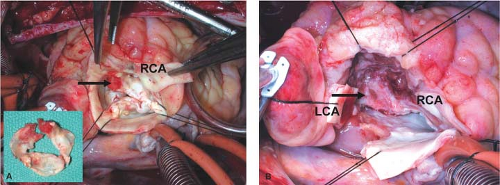 Figure 43.1 A: Invasion with wide cellulitis migrating outside aortic wall: Invasion might not be obvious. Infected aortic valve with rheumatic disease displaying small vegetations on right–left commissure (arrow). Immediate appearance did not suggest invasion or extra-aortic extension. B:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|