Surgery for Hypertrophic Cardiomyopathy
Joonhwa Hong
Hartzell V. Schaff
Indications
Previously, left ventricular outflow tract (LVOT) obstruction in hypertrophic cardiomyopathy (HCM) was thought to occur in the minority of patients and considered to have little prognostic impact on late survival. It is now known, however, that fixed or latent LVOT obstruction is present in almost 70% of symptomatic patients with HCM, and patients with resting gradients of 30 mm Hg or more have reduced late survival compared to patients with HCM and no LVOT obstruction. Guidelines for treatment of symptomatic patients with obstructive HCM recommend initial medical treatment with beta-blockers, verapamil, and/or disopyramide. For patients who remain symptomatic or are limited by side effects of medications, relief of LVOT obstruction should be considered because elimination of the gradient and associated mitral valve regurgitation will reliably relieve symptoms in over 90% of patients. Successful operation improves associated mitral regurgitation due to systolic anterior motion (SAM) of the mitral valve and improves pulmonary artery hypertension.
At present, the main indications for septal myectomy in patients with obstructive HCM are relief of symptoms and restoring diminished functional capacity. The issue of improving survival of patients with HCM by relief of gradients is unsettled. But the observational study by Ommen et al. does suggest that survival of patients following myectomy for obstructive HCM is similar to survival of HCM patients without obstruction and superior to survival of unoperated patients with obstructive HCM. The possible beneficial effect of septal myectomy on late survival is supported by other studies showing reduced incidence of implantable cardioverter defibrillator (ICD) discharges in patients following myectomy compared to medically treated patients. Septal myectomy may be considered as an alternative first line therapy in some patients with ideal anatomy, that is, those with isolated or predominant subaortic septal hypertrophy.
Most clinicians recognize the association between symptoms and fixed LVOT obstruction (gradient >30 mm Hg), but patients with lesser degrees of resting obstruction may be symptomatic because of high provocable gradients that become apparent with exercise or
other provocative maneuvers such as Valsalva, inhalation of amyl nitrite, or isoproterenol infusion during cardiac catheterization. Septal myectomy should be considered in this subgroup also, because relief of latent, provocable gradients produces the same degree of symptom relief as does myectomy in patients with fixed LVOT obstruction.
other provocative maneuvers such as Valsalva, inhalation of amyl nitrite, or isoproterenol infusion during cardiac catheterization. Septal myectomy should be considered in this subgroup also, because relief of latent, provocable gradients produces the same degree of symptom relief as does myectomy in patients with fixed LVOT obstruction.
Also, there is a subset of patients with apical HCM and diastolic heart failure. The apical variant of HCM is relatively uncommon and generally not associated with subaortic obstruction. But when present, symptoms of dyspnea and angina respond poorly to medical therapy. In some patients with apical HCM, a major contributor to diastolic dysfunction is a small left ventricular (LV) cavity. Apical myectomy to enlarge the left ventricle is indicated in patients with apical HCM and diastolic dysfunction due to small LV cavity size.
Contraindications
Contraindications to septal myectomy in patients with obstructive HCM are similar to risk factors for other types of cardiac surgery such as advanced age and frailty and concomitant illnesses that limit expected survival. In these patients, alcohol septal ablation (septal infarction) may be useful.
In most patients, hypertrophic obstructive cardiomyopathy is identified initially by transthoracic echocardiography, and important features are ventricular morphology and hemodynamics. The surgeon should identify the level of LV outflow obstruction and its relation to SAM of the mitral valve, as this will guide the extent of myectomy and surgical approach. In most patients, LVOT obstruction is in the subaortic area and can be identified on echocardiography by the bright endocardial scar that is the contact region with the SAM of the mitral leaflets. Some patients will have midventricular obstruction produced by contact of the anterolateral papillary muscle and the septum, and others may have multilevel obstruction. It is important to identify any intrinsic mitral valve abnormalities that may require direct repair at the time of myectomy. Echocardiography can also identify location and insertion of abnormal papillary muscles.
Cardiac magnetic resonance (CMR) imaging may be helpful in preoperative assessment in identifying ventricular morphology and SAM. Also, CMR with late gadolinium enhancement (LGE) can aid in assessing risk of sudden cardiac death. Chan et al. found LGE in 42% of HCM patients overall and in 70% of patients who had sudden cardiac death or were rescued from an event by defibrillation. In this study, more extensive LGE was associated with greater risk of sudden cardiac death events.
Chest pain is a common presentation in patients with HCM. Assessment of coronary arteries is indicated in patients who present with angina pectoris and those patients who are undergoing myectomy and have risk factors for coronary atherosclerosis. Coronary CT angiography (CCTA) has been used in patients with HCM and can detect coronary calcification, focal stenoses, and myocardial bridging. We have preferred coronary angiography as the most direct method of assessing coronary artery disease in patients with HCM who might be candidates for concomitant coronary artery bypass or unbridging of intramyocardial segments of the left anterior descending coronary artery.
Intraoperative Preparation
Operation for septal myectomy is performed under general anesthesia and routine monitoring lines are placed including a pulmonary artery catheter for pressure measurement and cardiac output determination. Intraoperative transesophageal echocardiography (TEE)
is helpful to confirm the cardiac anatomy and assess the mitral valve morphology and function and appearance of the ventricular septum. Most critical information is determined from the preoperative transthoracic echocardiogram, but the intraoperative images should be studied by the surgical team to confirm the thickness of the septum at the contact point to determine the length of septal obstruction, and to assess the LVOT gradient under anesthesia. To evaluate LVOT obstruction, the TEE probe is positioned as parallel as possible to the LVOT jet to obtain maximal Doppler velocities. The Doppler maximal instantaneous gradient will be higher than the peak-to-peak gradient at the same point in the same cardiac cycle. Also, the TEE may demonstrate papillary muscle abnormalities that were not apparent on the preoperative study.
is helpful to confirm the cardiac anatomy and assess the mitral valve morphology and function and appearance of the ventricular septum. Most critical information is determined from the preoperative transthoracic echocardiogram, but the intraoperative images should be studied by the surgical team to confirm the thickness of the septum at the contact point to determine the length of septal obstruction, and to assess the LVOT gradient under anesthesia. To evaluate LVOT obstruction, the TEE probe is positioned as parallel as possible to the LVOT jet to obtain maximal Doppler velocities. The Doppler maximal instantaneous gradient will be higher than the peak-to-peak gradient at the same point in the same cardiac cycle. Also, the TEE may demonstrate papillary muscle abnormalities that were not apparent on the preoperative study.
Bypass Setup and Pressure Measurement
For transaortic and transapical myectomy, a standard median sternotomy is the preferred approach. After administering heparin and placing the aortic inflow cannula, we measure intracardiac pressures in all patients. Although the Doppler-derived maximal instantaneous gradient may measure LVOT obstruction most accurately, in the operating room, peak-to-peak systolic gradient is the easiest to obtain, and the minimal underestimation of outflow gradient is clinically unimportant. The LVOT gradient is evaluated twice: Before myectomy 2 to 5 minutes before going on bypass and after hemodynamics are stabilized following myectomy.
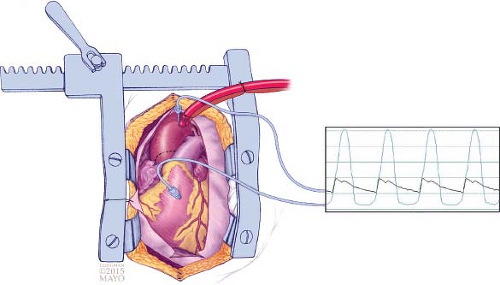
To measure the gradient in the operating room, a 2.5-inch, 22-gauge spinal needle is placed into the aorta close to the aortic cannula and another 3.5-inch, 22-gauge spinal needle is placed into the LV through the right ventricular free wall and septum (Fig. 4.1). Pressures from both needles are monitored and recorded simultaneously. To measure dynamic LVOT pressure gradient, a premature ventricular contraction is induced by tapping the heart and the higher gradient in the subsequent beat (Brockenbrough–Braunwald–Morrow sign) is recorded; in some patients, small doses of isoproterenol are administered to elicit a gradient. The needles are kept sterile for repeated pressure measurement after myectomy to confirm elimination of the LVOT gradient.
The right atrium is cannulated with a two-staged single venous cannula after hemodynamic assessment to avoid atrial arrhythmias during pressure measurements. We use normothermic cardiopulmonary bypass and maintain perfusion pressure ≥70 mm Hg. The aorta is clamped and cold blood cardioplegia is infused through an aortic tack vent;
we usually use an initial dose of 1,000 mL because of LV hypertrophy. If necessary, a repeat infusion of 400 mL of cardioplegia is infused directly in the left coronary ostium after 20 minutes of arrest.
we usually use an initial dose of 1,000 mL because of LV hypertrophy. If necessary, a repeat infusion of 400 mL of cardioplegia is infused directly in the left coronary ostium after 20 minutes of arrest.
Exposure
Excellent exposure of the subaortic septum is a critical step in performing an adequate septal myectomy. We use a transverse-oblique (hockey-stick) aortotomy beginning just cephalad to the commissure between the right and noncoronary sinuses (Fig. 4.1




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree