Congenital
Laryngomalacia
Vocal cord paralysis
Congenital subglottic stenosis
Subglottic hemangioma
Congenital laryngeal web
Laryngal atresia
Laryngotracheoesophageal cleft
Tracheomalacia
Bronchomalacia
Congenital tracheal stenosis
Tracheal agenesis
Acquired
Subglottic stenosis
Glottic stenosis
Tracheal stenosis
Bronchial stenosis
Congenital Laryngeal Anomalies
Laryngomalacia
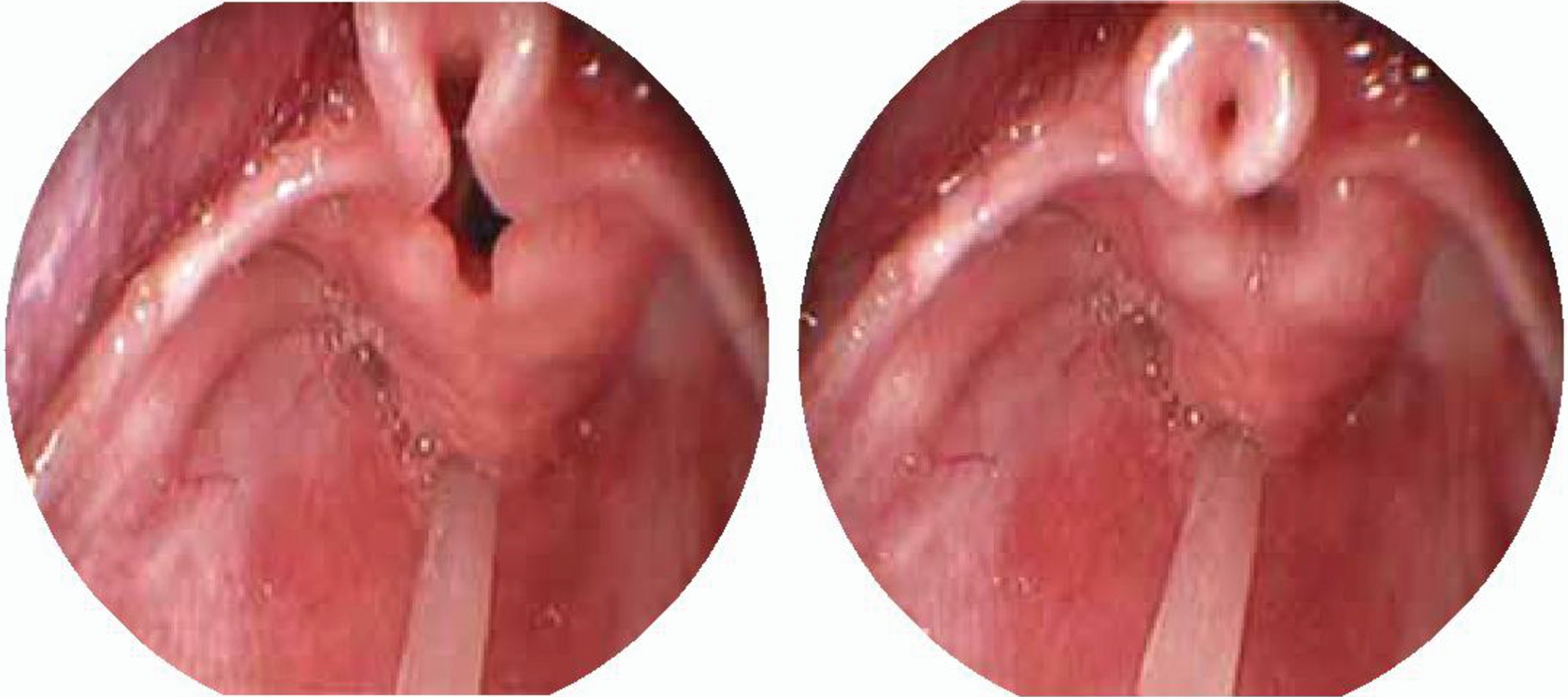
Laryngomalacia . Omega shaped epiglottis, short aryepiglottic folds. (a) Expiration, (b) Inspiration
Congenital Subglottic Stenosis
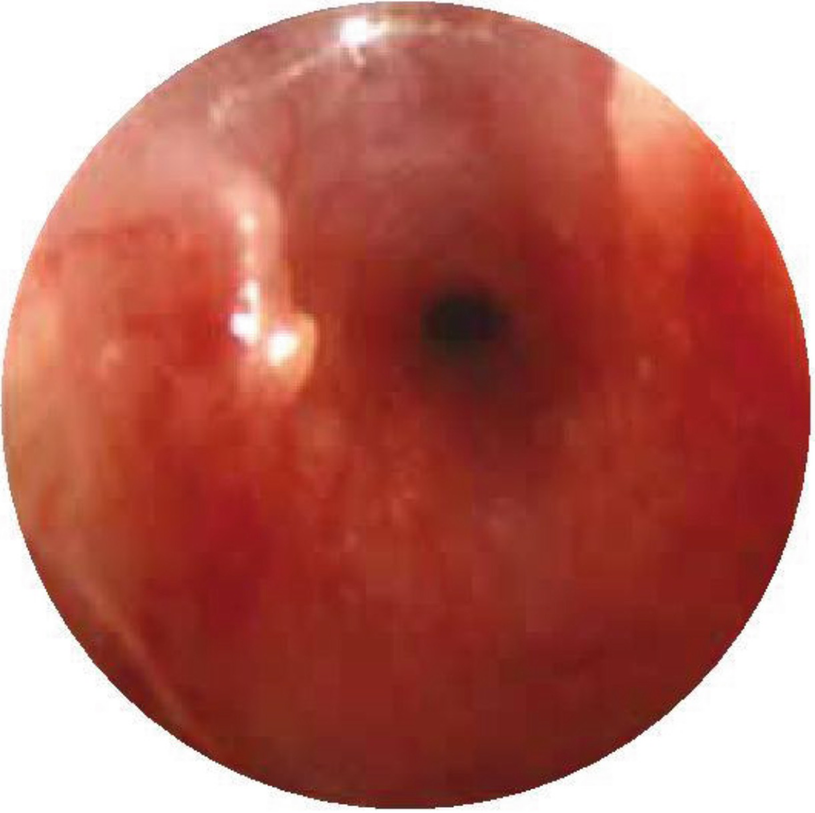
Subglottic stenosis. Direct laryngoscopy shows a severe narrowing of less than 1 mm of the subglottic space in a newborn with respiratory distress
Laryngeal Diaphragm or Webs
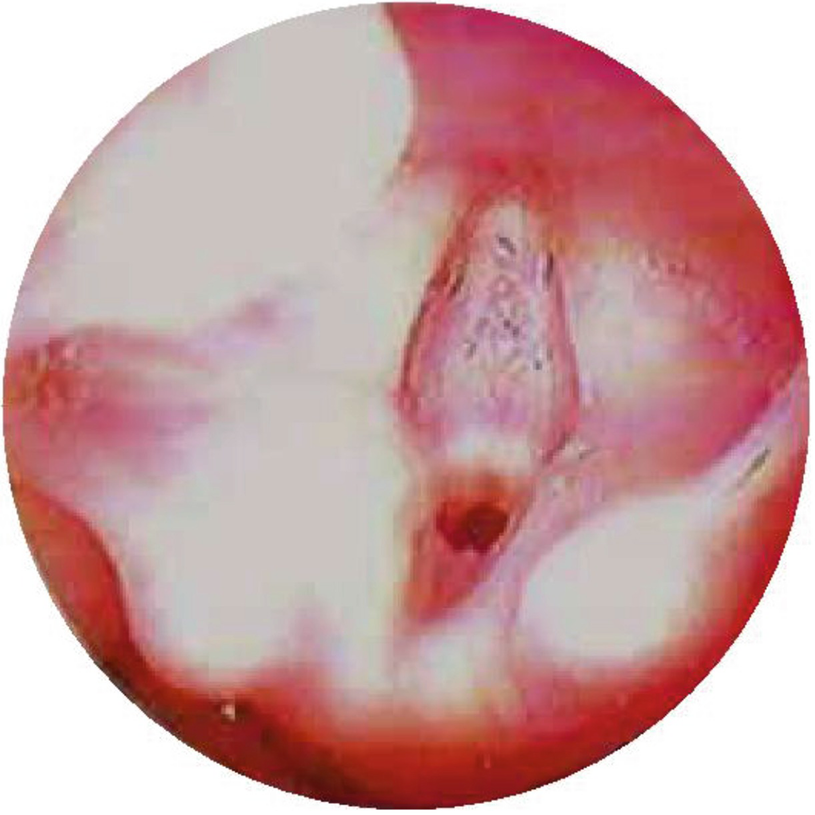
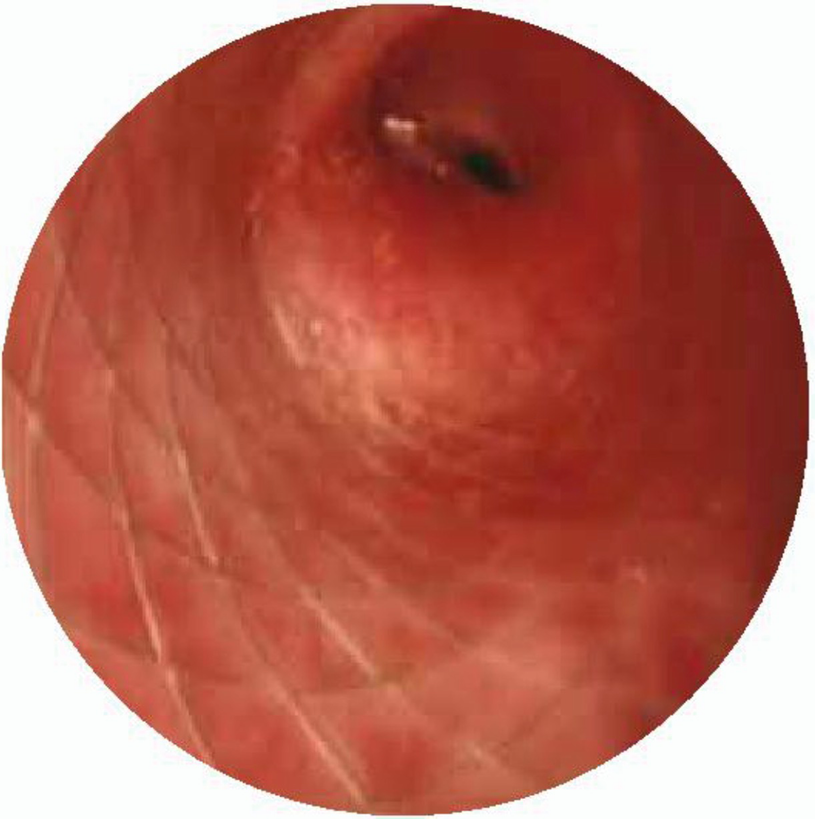
Laryngeal web. Type III laryngeal web with 75% of lumen obstruction
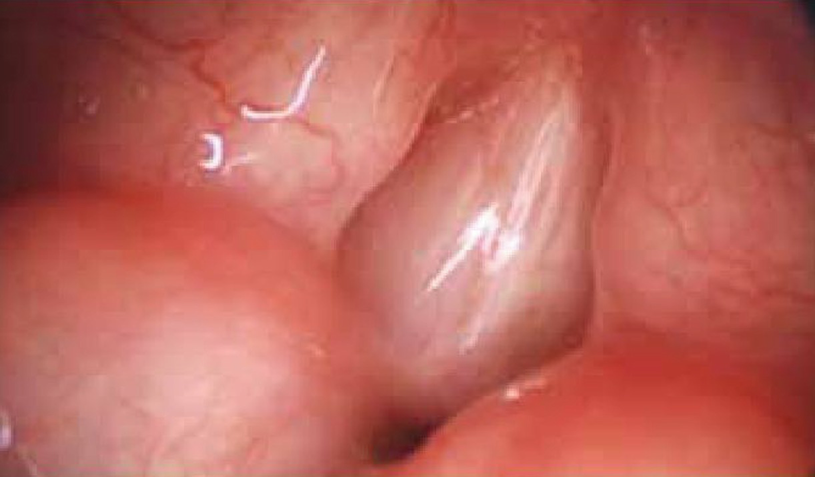
Laryngeal web. Laryngoscopy performed in a newborn with laryngeal web that shows 95% glottis obstruction and reduction of interarytenoid space
Initial diagnosis is made with a nasolaryngoscopy conducted while the patient is awake, which will rule out other possible diagnoses, such as laryngomalacia or vocal cord paralysis. A final evaluation requires direct laryngoscopy. Different therapeutic options are available: a newborn with laryngeal diaphragm diagnosis and severe airway obstruction during the first hours of life characteristically corresponds to a case of diaphragm with severe obstruction of the glottic space, and in many cases emergency surgery is required to secure the airway (tracheotomy). In patients with thin and anterior diaphragms, an endoscopic cut can be performed. In thick diaphragms, with mild to moderate symptoms, later repair is suggested, because it is easier in technical terms. In children with more severe symptoms, the intervention must be done early. To relieve obstruction, tracheotomy is also endorsed as an option while the child grows, in order to repair at a later age.
In the majority of type III and IV diaphragms, open surgery involves a two-stage repair that includes the division of the diaphragm and enlargement of the subglottic space. The current recommended technique consists of performing anterior laryngofissure with a siliconized laryngeal stent placement plus a temporary tracheotomy. The purpose of this laryngeal prosthesis is to allow an adequate epithelization of the medial border of the cords that was congenitally adhered and thus prevent them from adhering again. Between 6 and 8 weeks later, the laryngeal prosthesis is removed endoscopically, and then it is possible to remove the tracheotomy. As mentioned, it is advisable to expand the airway using an anterior or posterior graft of costal cartilage due to the frequent association that this pathology has with subglottic space stenosis. In grade III patients, having to enlarge the subglottic space in the posterior plate and the anterior wall occurs frequently. In mild forms, the approach is endoscopic surgery conducted by experts, and requires cutting off the web using a laser, and also a mucosal flap in the cords to prevent posterior synechiae and recurrence. The insertion of a keel endoscopically, between the medial borders of the separated cords, is another endorsed alternative in older patients.
Subglottic Hemangioma
It is a benign vascular tumor located in the subglottic space, specifically in the left posterolateral region, close to the vocal cord on the same side. It corresponds to 1.5%–3% of all benign lesions of the larynx, and its evolution is self-limited, starting to remit after 18 months. Hemangiomas of posterior localization, left and bilateral, are less common. In our experience, we have evaluated eight subglottic hemangiomas, one of them posterior bilateral associated with congenital narrowing of the subglottic space. Because initial treatment with propranolol was not enough, surgery had to be done, resecting the hemangioma, removing the cricoid posterior mucosa, and performing a partial cricoid resection and a primary thyrotracheal anastomosis without tracheotomy.
Currently, the treatment of choice is the administration of propranolol; however, there are other treatment options that are second-line therapy that should be analyzed in each patient, considering the size of the lesion, age, degree of obstruction, and response to steroids and propranolol. Observation is appropriate for older patients, in whom there is little or minimal associated symptomatology of respiratory obstruction.
In cases where airway obstruction caused by hemangioma is less than 50% of the lumen and the symptoms are mild, the alternative to observe and re-evaluate is valid. Endoscopic techniques of intralesional steroids injection, submucosal dissection with microdebrider, and laser ablation can only achieve partial removal of the lesion and cause some degree of damage to the mucosa or cricoid cartilage, so currently there is controversy regarding these therapies. Temporary tracheotomy offers a stable airway during the growth and involution phase of the hemangioma. In case of opting for this alternative, the presence of the stoma should be considered for a period of approximately 10–30 months. The advantage of this option is that the involution of the lesion is achieved without a surgical intervention that may be associated with cord injury. However, it should also be considered that tracheotomy in infants has associated morbidity and mortality and requires special care. Open surgical resection is the treatment of choice in symptomatic patients with large hemangiomas that grow quickly. It would also be indicated in patients in whom the involution of the hemangioma has not occurred within 2 years (and where a tracheotomy or observation was chosen). Endoscopic excision with CO2 laser, open resection, endoscopic resection with microdebrider, and tracheostomy are the main surgical alternatives. However, propranolol treatment currently prevails in most hemangiomas.
Congenital Tracheal Anomalies
Infrequent events, with an incidence of 1 per 60,000 live births. Most common congenital tracheal anomalies are tracheomalacia, bronchomalacia, tracheal cleft, and tracheal stenosis.
Tracheomalacia-bronchomalacia
Surgical alternatives, such as aortopexy, bronchopexy, intraluminal supports (stents), partial resections, and tracheotomy are reserved for severe cases: apneas, respiratory obstruction episodes during feeding, and recurrent respiratory infections.
Indicated when there is a collapse greater than 50% of the tracheal lumen. It consists of a suspension of the anterior wall of the aortic arch to the internal wall of the sternal manubrium in order to increase the space of the mediastinum, so that the malacic airway segment is free of compression by the vascular structures that surround it.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


