SURFACTANT THERAPY AND NEONATAL HEMODYNAMICS
Introduction
Respiratory distress syndrome (RDS) due to surfactant deficiency or dysfunction leads to hypoxia, hypercarbia, acidosis, myocardial dysfunction, and pulmonary hypertension. RDS occurs in 40% to 50% of preterm infants born before 32 weeks of gestational age (GA).1–3 Hypoxemic respiratory failure (HRF) is one of the most common problems in newborn infants, affecting 2% of all live births. Persistent pulmonary hypertension (PPHN) often complicates HRF. The overall incidence of PPHN is 2 to 3 per 1000 live births.4,5 In newborns with surfactant deficiency, normal cardiopulmonary transition fails to occur, leading to HRF and PPHN. In preterm infants with RDS due to developmental deficiency of surfactant production, pulmonary vascular pressures are elevated to systemic or subsystemic levels.6,7 In term or late-preterm infants (340/7–360/7 weeks) with HRF, PPHN occurs in 10% of these infants. Major changes during the fetal to neonatal transition include iso-volumic transformation of a fluid filled lung to an air-breathing unit, expansion of the lung, almost 4- to 6-fold increase in pulmonary blood flow (PBF) after birth, and precipitous drop in pulmonary vascular resistance (PVR).8 In newborns with lung disease, this drop in pulmonary pressure is delayed, leading to a prolonged transition phase.
Preterm infants with RDS who subsequently develop bronchopulmonary dysplasia (BPD) also have PPHN, secondary to alveolar and vascular hypoplasia. Pathogenesis of BPD is multifactorial. Ventilator-induced lung injury due to volutrauma, oxidant injury due to supplemental oxygen therapy, propensity of preterm infants to release proinflammatory cytokines, such as tumor necrosis factor-alfa (TNF-α), interleukins (IL-1β and IL-8), and inability to produce anti-inflammatory cytokine, such as, IL-10 resulting in a persistent inflammatory state.9,10 This imbalance leads to abnormal or aberrant repair of the developing lungs.
Respiratory failure due to RDS is a common component of cardio-respiratory dysfunction in preterm and term infants, and management often includes surfactant and hemodynamic support. There is a delicate and interdependent interaction between the pulmonary and cardiovascular systems in preterm infants with RDS. Surfactant therapy and respiratory management have consistently led to improved neonatal hemodynamics when applied appropriately.
Management of RDS
Antenatal
Antenatal steroids (AS) given to pregnant women between 24 and 34 weeks of gestation lead to global maturation of the fetus, including cardiorespiratory system.11–14 Cardiopulmonary effects include improvement in cardiac function, maintenance of higher blood pressures during the first 3 days after birth in preterm infants, less need for inotropic support, and thinning of pulmonary interstitium, improved lung compliance, and increase in surfactant synthesis as well as release of preformed surfactant into the alveolar spaces. Biochemical changes tend to dissipate within a week of AS administration; however, it is not known as to how long the structural pulmonary changes persist. AS use has been shown to significantly decrease the incidence of RDS, mortality, and intraventricular hemorrhage (IVH). AS exposure followed by postnatal surfactant therapy has been shown to have additive effects in improving outcomes.15–17
Postnatal
Following preterm birth, surfactant therapy improves oxygenation, decreases air leaks, and improves neonatal and infant survival.18–20 Surfactant therapy results in improvement in ventilation to perfusion mismatch, decrease in PCO2, and increase in lung compliance. Pulmonary vascular pressures are high both at low- and high-lung volumes. Pulmonary vascular pressures are also reduced in postsurfactant therapy because of recruitment of the saccules/alveoli and by optimizing lung volumes. Furthermore, surfactant treatment has also been shown to be beneficial in newborn patients with surfactant inactivation, such as, in neonates with meconium aspiration syndrome (MAS), congenital or acquired pneumonia, pulmonary hemorrhage, and/or neonatal sepsis-induced systemic inflammatory response syndrome. In late preterm or term infants with HRF, treatment with surfactant decreases the need for extracorporeal membrane oxygenation (ECMO).
Surfactant Therapy in Preterm Infants
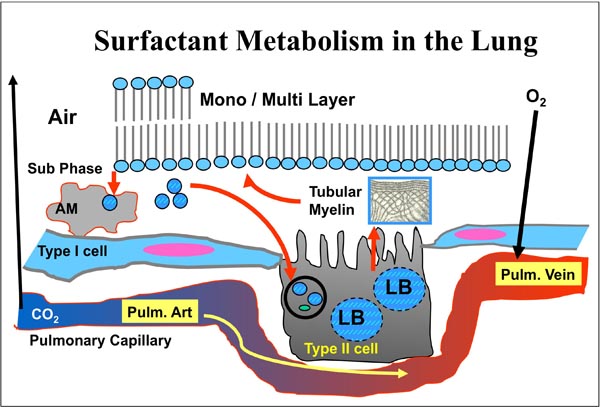
Surfactant is a complex mixture of lipids and proteins, synthesized by type II pneumocytes and released into the alveolus by a process of exocytosis (Figure 23.1). To optimize and improve gas exchange in alveoli of mammalian lungs, surface tension of the alveolar epithelium is reduced significantly by pulmonary surfactant, a mixture of lipids and 4 different proteins, namely surfactant protein (SP)-A, -B, -C, and -D.21–24 Major lipid component is dipalmitoylphosphatidylcholine, which along with SP-B adsorbs as a mono- or multilayer at the air–liquid interphase and reduces surface tension. Properties other than lowering of surface tension, such as spreading and viscosity are also important for undisturbed surfactant function.25
Figure 23.1. Surfactant metabolism in the lung and gas exchange at the alveolar-capillary membrane. LB, lamellar body; AM, alveolar macrophage.
Preterm infants with RDS have decreased lung compliance, lower functional residual capacity, and end-expiratory atelectasis due to high surface tension, resulting in ventilation–perfusion mismatch, hypoxemia, and hypercarbia. As a result of lower lung volumes, pulmonary pressures are increased. Exogenous surfactants derived from animal sources as well as synthetic surfactants have been extensively evaluated in preterm infants with RDS.26 At the preset time, animal-derived surfactants are better than synthetic surfactants for early and selective surfactant treatment of RDS.27 Although the early trials of prophylactic surfactant therapy compared with the selective use of surfactant for RDS demonstrated a decreased risk of air leak and mortality, recent large trials that reflect current practice, including greater utilization of AS and routine postdelivery stabilization on continuous positive airway pressure (CPAP), demonstrate less risk of chronic lung disease or death when using early stabilization on CPAP with selective surfactant administration to infants requiring intubation.28
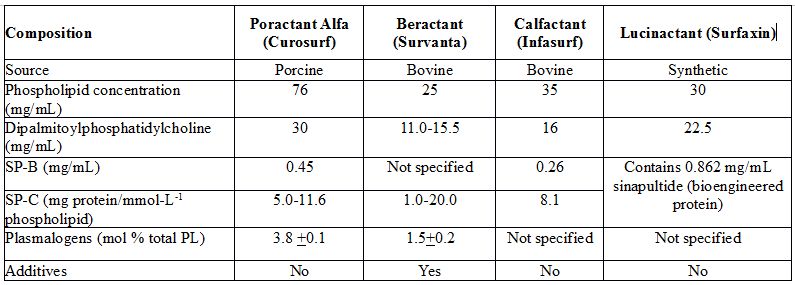
Synthetic surfactant that is recently licensed in the United States has not been evaluated for selective treatment or after nasal CPAP stabilization in the delivery room. Animal-derived surfactants that are commonly used worldwide include 2 bovine-derived preparations, namely, beractant and calfactant, and a porcine-derived surfactant, namely, poractant alfa. Composition of these surfactants is shown in Table 23.1. There are major biochemical differences between these 3 surfactants. Porcine-derived surfactant, poractant alfa, contains the highest amount phospholipids distributed in the smallest volume, and has highest amount of antioxidant phospholipid, plasmalogen as well as high amounts of surfactant-associated protein, SP-B. Experimental studies suggest that the main role of a low surface viscosity is to allow a more homogeneous distribution of densely packed lipids during expiration. To minimize surface viscosity, plasmalogens, a minor surfactant component, may have a major influence on the surface viscosity of phospholipid mixtures.25
Table 23.1. Composition of surfactants
Randomized, controlled trials comparing bovine surfactants have shown no difference in any of the outcomes (Table 23.2).29,30 However, comparisons of beractant versus poractant alfa have shown that porcine surfactant is better than bovine surfactant with respect to rapid improvements in oxygenation, faster weaning of peak inspiratory pressures (PIPs) and supplemental oxygen, less need for additional doses, less patent ductus arteriosus (PDA), more BPD free survival, shorter length of stay (LOS), and lower mortality with the use of 200 mg/kg of poractant alfa (Table 23.3).31–40 Use of higher doses has been shown to be associated with less air leaks, less BPD, less IVH, longer duration of action, longer half life, faster weaning, and less need for additional doses.41–45 Based on evidence from randomized, controlled, clinical trials, European consensus guidelines recommend the use of 200 mg/kg dose of poractant alfa as the initial dose, which was also endorsed by the European Association of Perinatal Medicine.46
Table 23.2. Clinical trials comparing bovine derived surfactants: Beractant versus calfactant
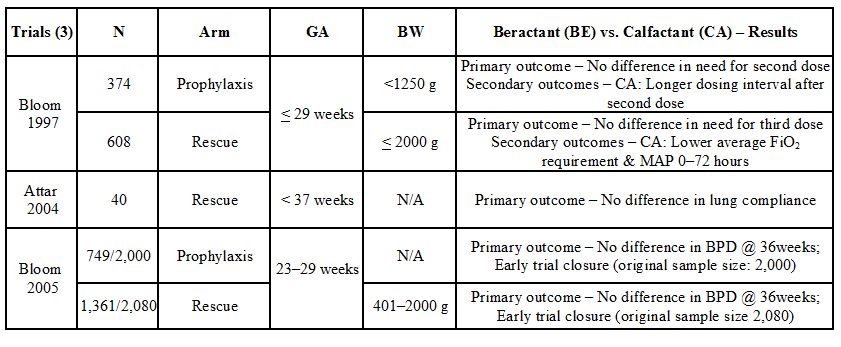
Table 23.3. Principal currents involved in the sinus node action potential
| Trials (6) | N | Type | BW or GA | Poractant Alfa (PA) Versus Beractant (BE)—Results |
| Speer 1995 | 73 | Tx | 700 to 1500 g | PA: Lower FiO2, PIP and MAP @ 12 to 24 hours |
| Baroutis 2003 | 80 | Tx | <2000 g | PA: Fewer days on O2 and PPV; decreased LOS |
| Ramanathan 2004 | 293 | Tx | 750 to 1750 g | PA: Lower FiO2, fewer doses, decreased mortality <32 weeks |
| Malloy 2005 | 58 | Tx | <37 weeks | PA: Lower FiO2 up to 48 hours, fewer doses, lower volume |
| Fujii 2010 | 52 | Tx | <30 weeks | PA: Faster weaning, less air-leaks, PDA and MV at 72 hours |
| Dizdar 2011 | 126 | Tx | <37 weeks | PA: Faster weaning, fewer doses, rapid extubation and higher survival without BPD |
BW, birth weight; GA, gestational age; PIP, peak inspiratory pressure; MAP, mean airway pressure; PPV, positive pressure ventilation; LOS, length of stay; Tx, rescue treatment; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia
Surfactant Treatment in Larger and Mature Preterm Infants
Compared with controls with similar weights and gestations, larger and more mature surfactant-treated infants had a lower incidence of death (3.4% vs 6.7%; relative risk [RR]: 0.56;95% confidence interval [CI]: 0.35–0.88; number needed to treat [NNT]: 33), and BPD (5.8% vs 10%; RR: 0.57; 95% CI: 0.40–0.81; NNT: 25).47–49 However, these studies were done when AS use was low and stabilization in the delivery room with CPAP was not routinely employed. Current recommendations include surfactant treatment in more mature and/or late-preterm infants (>34 weeks GA at birth) presenting with HRF.
Surfactant Therapy and Nasal Intermittent Positive Pressure Ventilation (NIPPV)
Despite more than 3 decades of using surfactant for RDS in preterm infants, BPD rate remains unacceptably high. Studies with nasal CPAP and surfactant therapy when compared with invasive ventilation and surfactant have shown similar rates of death or BPD as a composite outcome.50 However, preterm infants with RDS managed on NIPPV postsurfactant therapy have a significantly lower risk for BPD.51–53 Large studies combining surfactant and NIPPV are urgently needed. Bradycardia and reflux of the medication during administration were also lower with poractant alfa due to lower viscosity and smaller volume, when compared with calfactant.54 Furthermore, cerebral blood flow velocity is not affected during the administration of poractant alfa as compared with beractant.55
Surfactant Treatment and PDA
The ductus arteriosus (DA) is an important fetal channel during fetal life and directs the deoxygenated blood away from the lungs into the descending aorta and the placental circulation via the umbilical arteries for placental gas exchange (see Chapter 1). At birth, closure of the DA is an important event during the postnatal cardiopulmonary adaptation. Incidence of PDA is inversely proportional to GA. In infants <28 weeks GA, 60% of the preterm neonates have a PDA.56 In the extremely low GA newborns, reported incidence of PDA ranges between 30% and 60%. GA, AS exposure, RDS, and Caucasian race have been identified as independent risk factors for a persistent DA and degree of ductal patency.57 Persistent PDA has been associated with significant mortality and morbidities.58,59 Surfactant administration acutely increases the alveolar pool size, lowers surface tension and reverses the blood gas abnormalities. With improved oxygenation, PVR decreases more rapidly, and an increase in blood flow through PDA is often observed.60 Some of these changes in hemodynamics may be minimized by using small surfactant volumes, maintaining delivery of normal tidal volume using volume guarantee, volume targeted mode of ventilation, and/or rapidly weaning supplemental oxygen.55 Clinical signs of left-to-right shunting through a PDA may occur earlier in very preterm infants postsurfactant therapy.61–63 However, none of the randomized, placebo-controlled, clinical trials using surfactant have shown an increase in hemodynamically significant PDA (hsPDA). Interestingly, in a comparison study between beractant and poractant alfa, Fujii et al demonstrated a lower incidence of hsPDA in the poractant alfa-treated group.35
PDA adversely impacts systemic as well pulmonary circulations. Systemic effects include decrease in diastolic blood flow velocity,64,65 retrograde diastolic flow and increase in pulsatility index,66,67 and increase in cerebral oxygen extraction.68 Increase in PBF postsurfactant therapy has also been reported.69,70 Cerebral hemodynamic changes due to PDA in preterm infants with RDS have been implicated in the pathogenesis of intraventricular hemorrhage. This view is strengthened by the fact that in a meta-analysis of 14 trials using prophylactic indomethacin treatment to close the PDA significantly decreased the incidence of IVH.71 Left ventricular output (LVO) is increased in patients with hsPDA, and despite this increase in LVO, flow in the abdominal aorta is decreased.72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree