Essentials of Diagnosis
Supraventricular tachycardias (SVTs) are rapid rhythm disturbances originating from the atria or the atrioventricular (AV) node. In the absence of a bundle branch block, there is intact conduction to the ventricles via the right and left bundles leading to a narrow and normal-appearing QRS. Therefore, these arrhythmias are also often called narrow complex tachycardias. Since many of the SVTs are episodic, many clinicians also refer to this group of arrhythmias as paroxysmal SVTs. Radiofrequency ablation has become an important therapeutic option in the management of SVTs because of its ability to cure these arrhythmias safely. Table 11–1 outlines the pharmacologic therapy for SVTs.
Agent | Indication | Intravenous Dose | Oral Dose | Adverse Effects | Drug Interactions |
|---|---|---|---|---|---|
Class Ia | |||||
| Quinidine | AF, AFL, AVNRT, AVRT | 6–10 mg/kg over 20–30 min | 200–400 mg q4–6h; q8h with long-acting preparations | Hypotension (especially IV), ventricular proarrhythmia, GI disturbance, thrombocytopenia | ↑ Digitalis level ↑ Warfarin effect ↑ Metoprolol, propranolol, propafenone levels |
Procainamide | AF, AFL, AVNRT, AVRT | Bolus: 15 mg/kg given as 20 mg/min Infusion: 2–4 mg/min | 50 mg/kg/day q3–4h; twice daily dosage with long-acting preparation | GI disturbance, hypotension, SLE, agranulocytosis, FUO hemolytic anemia, myasthenia gravis aggravation, ventricular proarrhythmia | ↑ Procainamide level with cimetidine, quinidine, and amiodarone |
Class 1c | |||||
Flecainide | AF, AFL, AT, AVNRT, AVRT | N/A | 50–200 mg q12h | Ventricular proarrhythmia, CHF, GI disturbance, CNS (dizziness, tremor, light-headedness) | ↑ Digitalis level ↑ Flecainide level with amiodarone, cimetidine, Norpace, propranolol ↓ Flecainide level with smoking |
Propafenone | AF, AFL, AVNRT, AVRT | N/A | 150–300 mg q8h or 225–425 mg bid (long-acting form) | GI disturbance, CNS (dizziness), metallic taste, CHF, first-degree AVB, IVCD, positive ANA | Synergism with β-blockers |
Class II (IV) | |||||
Esmolol | Ventricular rate control for AF, AFL, ST, AT | Bolus: 500 mcg/kg over 1–2 min Infusion: 50–200 mcg/kg/min | N/A | CHF, AVB, bradycardia, bronchospasm | |
Propranolol | Ventricular rate control for AF, AFL, ST, AT | 1–5 mg at 1 mg/min | 20–320 mg/day q6h, q8h, q12h, or once daily, depending on preparation | CHF, AVB, bradycardia, bronchospasm | |
Class III | |||||
Sotalol | AF, AFL, AVNRT, AVRT, AT | N/A | 80–160 mg q12h | Dyspnea, fatigue, dizziness, CHF, bradycardia, ventricular proarrhythmia, bronchospasm | Synergism with Ca2+ antagonists or β-blockers |
Amiodarone | AF, AFL, AVNRT, AVRT, AT | Bolus: 150 mg over 10 min Infusion: 1 mg/min × 6 h, then 0.5 mg/min | 100–400 mg once daily | Pulmonary toxicity, CHF, tremor, bradycardia, ↑ LFTs, corneal deposits, skin discoloration, GI intolerance, hyper-/hypothyroidism | ↑ Digoxin levels ↑ Warfarin effect ↑ Quinidine, procainamide/NAPA, flecainide ↑ Phenytoin level |
Ibutilide | AF, AFL | 1 mg bolus over 10 min; second bolus, if needed, after 10-min wait | N/A | Ventricular proarrhythmia, hypotension, GI disturbance | |
Dofetilide | AF, AFL | N/A | 125–500 mcg twice daily modified by algorithm | Ventricular proarrhythmia, headache, chest pain, nausea, dizziness | Contraindicated with verapamil, cimetidine, ketoconazole, trimethoprim |
Class IV | |||||
Diltiazem | AF, AFL, AVNRT, AVRT, AT, MAT | Bolus: 0.25 mg/min over 2 min then 0.35 mg/kg in 15 min if needed Infusion: 5–15 mg/h | 90–360 mg/day in 1–4 divided doses, depending on preparation | Hypotension, bradycardia, CHF, AVB | Synergism with β-blockers |
Verapamil | AF, AFL, AVNRT, AVRT, AT, MAT | 2.5–20 mg over 20 min in divided doses | 40–120 mg q8h; 240–360 mg once daily of long-acting preparation | Hypotension, bradycardia, CHF, AVB | Synergism with β-blockers |
Class V | |||||
Adenosine | SVT diagnosis, AVNRT, AVRT, AT termination | 6 mg IV rapid bolus followed by 12 mg × 2 if needed; half dosage if administered in central line | N/A | Chest tightness, facial flushing, dyspnea, AVB | ↑ Activity by dipyridamole ↓ Activity by theophylline |
Digoxin | Ventricular rate control for AF, AFL, AT (generally not very effective in active patients) | Up to 1.0 mg bolus in divided doses followed by 0.125–0.375 mg/day | 0.125–0.375 mg/day in single dose | GI disturbance, conduction defects, atrial/ventricular arrhythmias, headache, visual disturbances | ↑ Digoxin level: amiodarone, quinidine, verapamil, indomethacin, spironolactone, alprazolam, erythromycin, tetracycline ↓ Digoxin level: antacids, cholestyramine, rifampin, neomycin ↑ Risk of digitalis toxicity with potassium-depleting diuretics |
Arrhythmias occur as a result of three main mechanisms: reentry, which is most common; enhanced or abnormal automaticity; and triggered activity.
Reentrant arrhythmias sustain themselves by repetitively following a revolving pathway comprising two limbs, one that takes the impulse away from and one that carries it back to the site of origin. For reentry to exist, an area of slow conduction must occur, and each limb must have a different refractory period (see the discussion on AV nodal reentrant tachycardia). In this situation, pacing (by inducing refractoriness in one limb of the circuit) can typically initiate a reentrant tachycardia. Once established, pacing can also terminate the tachycardia by interfering with impulse propagation in one of the limbs.
The second mechanism, automaticity, refers to spontaneous and, often, repetitive firing from a single focus, which may either be ectopic or may originate in the sinus node. It should be noted that automaticity is an intrinsic property of all myocardial cells. This mechanism comprises two subcategories. Enhanced automaticity is defined as a focus that fires spontaneously and may originate in the sinus node, subsidiary pacemakers in the atrium including the Eustachian ridge, Bachmann bundle, coronary sinus and AV valves, the AV node, His-Purkinje system, and the ventricles. Abnormal automaticity is usually secondary to a disease process causing alterations in ionic flow that produces a lower (ie, more positive) resting diastolic membrane potential. Threshold potential is therefore more easily attained, thereby increasing the probability of a sustained arrhythmia.
The third mechanism, triggered arrhythmias, depends on oscillations in the membrane potential that closely follow an action potential. In the absence of a new external electrical stimulus, these oscillations, or after-depolarizations, cause new action potentials to develop. Thus, each new action potential results from the previous action potential. These arrhythmias can be produced by early or late after-depolarization, depending on the timing of the first after-depolarization relative to the preceding action potential (the one that spawned the triggered activity). In early after-depolarizations, membrane repolarization is incomplete, which allows an action potential to be initiated by a subthreshold stimulus. This type is often associated with electrolyte disturbance and may be the mechanism responsible for arrhythmogenesis related to the prolonged QT syndrome and torsades de pointes caused by quinidine. With delayed after-depolarization, membrane repolarization is complete, but an abnormal intracellular calcium load causes spontaneous depolarization. The reason for the high calcium levels is unclear, but it can be related to inhibition of the sodium pump by drugs such as digoxin. In either type of arrhythmia, the process may be repetitive and lead to a sustained tachycardia.
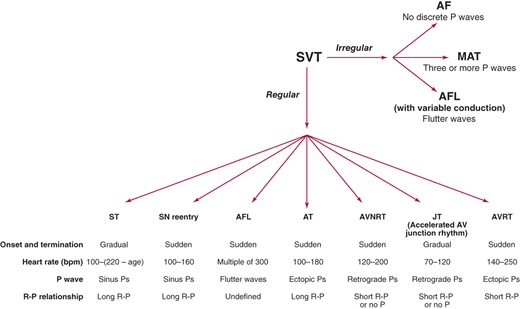
A systematic approach to interpreting the 12-lead electrocardiogram (ECG) will allow accurate determination of the type of SVT in most cases (Figure 11–1). The first step is to determine whether the rhythm is regular or irregular. If it is irregular, the rhythm is likely either atrial fibrillation, atrial flutter with variable conduction, or multifocal atrial tachycardia (MAT). The appearance of the P waves or lack of P waves will usually distinguish between these three entities. In atrial fibrillation, there is chaotic atrial activity. In atrial flutter, P waves are seen at rate of 240–320 bpm. In MAT, there are P waves preceding each QRS complex, and there are at least three different P-wave morphologies.
Figure 11–1.
Algorithm for distinguishing supraventricular tachycardias. AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reciprocating tachycardia; JT, junctional tachycardia; MAT, multifocal atrial tachycardia; SN, sinus node; ST, sinus tachycardia; SVT, supraventricular tachycardia.
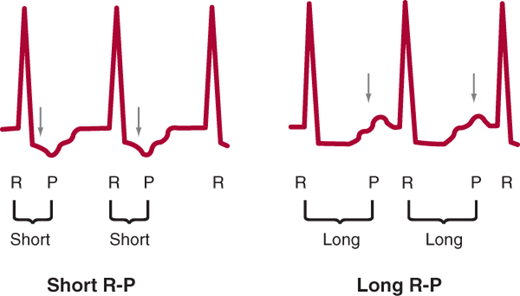
If the SVT is regular, there are several main types of SVT to consider. The SVT could be sinus tachycardia, sinus node reentry, atrial flutter, atrial tachycardia, AV nodal reentrant tachycardia (AVNRT), junctional tachycardia, or atrioventricular reciprocating tachycardia (AVRT). The type of regular SVT can be usually identified by examining four aspects of the 12-lead ECG: onset and termination, heart rate, P wave morphology, and R-P relationship. Sinus tachycardia and junctional tachycardia typically have very gradual onset, whereas the other SVTs usually start and stop more suddenly. Rate can also be helpful since sinus tachycardia cannot typically go over 220-age bpm and the heart rate in atrial flutter is often a multiple of 300. P-wave morphology can be helpful since retrograde P waves (negative in the inferior leads: II, III, and aVF) favor AVNRT and junctional tachycardia. Finally, R-P relationship refers to the distance from the R wave to the next P wave during tachycardia. If this distance is longer than the P-R interval, the SVT is termed “long R-P,” whereas if this distance is short, it is termed “short R-P” (Figure 11–2).
Sinus Tachycardia & Sinus Node Reentry
- Onset and termination: gradual.
- Heart rate: 100 to (220 – age) bpm.
- P wave: identical to normal sinus rhythm P wave.
- R-P relationship: long.
When the sinus node fires at a rate of more than 100 bpm, the rhythm is, with one exception, considered sinus tachycardia (see later section, Sinus Node Reentry). The onset and termination of sinus tachycardia are invariably gradual. The range for heart rate in sinus tachycardia is 100 to (220 – age) bpm; faster rates usually imply a different cause. Confirming that the tachycardic P waves are identical in morphology and axis to the normal sinus rhythm P waves is essential to the diagnosis. Like normal sinus rhythm, the R-P relationship in sinus tachycardia is typically long R-P, unless the patient has a very long P-R interval, which can usually be seen on the baseline ECG.
Sinus tachycardia is usually a physiologic response, activated when the body requires a higher heart rate to meet metabolic demands or maintain blood pressure. Common causes are exercise, hypotension, hypoxemia, heart failure, sepsis, fever, hyperthyroidism, fluid depletion, and blood loss.
The heart rate achieved is proportional to the intensity of the stimulus, but the rapidity with which the heart rate increases and decreases is a function of how quickly the stimulus is applied and withdrawn.
Vagal maneuvers slow the tachycardia gradually but only while being performed; when the vagal stimulus is removed, the heart rate gradually returns to where it started.
Attempting to slow the heart rate pharmacologically can be detrimental because it counteracts the compensatory mechanism provided by the tachycardia. Therefore, management is usually focused on treating the underlying cause of the sinus tachycardia.
- Onset and termination: sudden.
- Heart rate: 100–160 bpm.
- P wave: identical to normal sinus rhythm P wave
- R-P relationship: long.
This uncommon rhythm accounts for less than 5% of SVTs. It uses the sinus node or perinodal tissue as a critical part of the reentrant circuit, producing P waves identical to those seen during normal sinus rhythm. The heart rate usually falls between 100 and 160 bpm. Like sinus tachycardia, the R-P relationship is typically long R-P. Unlike sinus tachycardia, sinus node reentry is initiated by an ectopic beat rather than a physiologic stimulus and possesses the characteristics typical of a reentrant circuit. It therefore begins and ends abruptly and responds to vagal maneuvers and pharmacologic interventions by terminating rather than slowing.
The arrhythmia can be terminated quickly with intravenous adenosine, verapamil, or diltiazem, or via carotid massage. Long-term treatment uses β-blockers and calcium channel blockers. The largest reported series of patients treated with catheter ablation described success in all 10 patients. No complications were reported. Other smaller series found similar efficacy.
Atrial Flutter
- Onset and termination: sudden.
- Heart rate: usually a multiple of 300.
- P waves: flutter waves at 250–340 bpm.
- R-P relationship: undefined due to flutter waves.
- Prominent neck vein pulsations of about 300/min.
Atrial flutter is usually associated with organic heart disease and is second in frequency only to atrial fibrillation in post–coronary bypass surgery patients, with an incidence of up to 33%. With a typical atrial rate of 300 bpm (range 250–340 bpm), atrial flutter usually produces a “sawtooth” appearance (F waves). As is the case with atrial fibrillation (see Chapter 12), the ventricular rate depends on conduction through the AV node. Unlike atrial fibrillation, the ventricular impulses are transmitted at some integer fraction of the atrial rate. In rare circumstances, 1:1 conduction may occur. Fixed 2:1, 3:1, or 4:1 block is the usual scenario. However, variable block can also occur, leading to one of the three types of irregular SVTs (see Figure 11–1). If flutter is suspected but F waves are not clearly visible, vagal maneuvers or pharmacologic agents, such as adenosine, can help unmask the flutter waves by enhancing the degree of AV block.
Atrial flutter occurs in a variety of forms; the most common is isthmus-dependent counterclockwise atrial flutter; followed by the isthmus-dependent clockwise atrial flutter; and then the atypical, nonisthmus-dependent variety. The counterclockwise flutter is recognized electrocardiographically by negative F waves in leads II, III, and aVF and positive F waves in lead V1. The single reentrant wavefront proceeds up the interatrial septum, across the roof of the right atrium, down the lateral wall, and across the inferior wall. Clockwise flutter, on the other hand, has positive F waves in leads II, III, and aVF and negative F waves in lead V1. The reentrant circuit in this case moves in the reverse direction. In both of these types of atrial flutter, the atrial rates range between 250 and 340 bpm.
Symptoms attributable to atrial flutter are secondary to the ventricular response in addition to any underlying cardiac diseases. Dizziness, palpitations, angina-type chest pain, dyspnea, weakness, fatigue, and, occasionally, syncope may be the presenting symptoms. In those patients with poor left ventricular function, overt congestive heart failure may ensue.
Clinical evaluation is similar to that described for atrial fibrillation (see Chapter 12), but underlying heart disease is detected more often with atrial flutter than with fibrillation.
Several antiarrhythmic agents can prevent recurrences of atrial flutter. It appears that both class Ia and Ic agents are effective. Class III agents, such as sotalol and amiodarone, can also work very well. Dofetilide, a newer class III agent, which blocks the rapid form of the delayed rectifier current, Ikr, has also been found effective in converting to and maintenance of sinus rhythm. Its administration requires initiation in the hospital and an ECG-monitored setting. Drugs that are contraindicated with its use include verapamil, ketoconazole, cimetidine, trimethoprim, prochlorperazine, megestrol, and hydrochlorothiazide. With regard to safety, dofetilide has an overall proarrhythmic event rate of approximately 0.9%, which is less than the 3.3% seen in patients with congestive heart failure or the 2.5% in patients with previous ventricular tachycardia.
It should be emphasized that an AV nodal blocking agent should be started before initiating a class I drug. If the AV node is unblocked, a type I agent could facilitate conduction of atrial flutter by improving nodal conduction or by slowing the flutter rate and paradoxically increasing the ventricular response.
Once the diagnosis of atrial flutter is made, assessment of the patient’s status will dictate whether to perform cardioversion immediately. Immediate cardioversion can be accomplished with synchronized direct-current (DC) cardioversion, rapid atrial pacing to interrupt the macroreentrant circuit, or intravenous infusion of an antiarrhythmic agent. For DC cardioversion, as little as 25 J may be all that is required; however, at least 50 J is recommended to avoid extra shocks, and 100 J will terminate almost all episodes of atrial flutter. The major drawback with DC cardioversion is the need to administer sedation.
Rapid atrial pacing is another method that may terminate the arrhythmia. Pacing is best performed in the right atrium at a rate faster than the flutter rate, which allows the circuit to be entered by the pacing impulse. If the extrinsic pacing rate exceeds the rate that can be sustained through the zone of slow conduction, the flutter wavefront can be interrupted and will no longer be present when the pacing is stopped. If the patient has a pacemaker or implantable cardiac defibrillator with an atrial lead, pace termination can be done painlessly via the device. An alternative method uses a swallowed transesophageal electrode. Because of the interposed tissue, a high current is often necessary to capture and pace the atrium reliably, which may cause significant discomfort to the patient. Of note, overdrive pacing may precipitate atrial fibrillation, which usually terminates spontaneously after several minutes. Should the atrial fibrillation persist, however, control of the ventricular response rate is typically easier when compared with atrial flutter.
Finally, rapid pharmacologic cardioversion can be considered with intravenous agents such as ibutilide. Ibutilide is a unique class III antiarrhythmic agent with a rate of conversion of approximately 60% in patients with atrial flutter of less than 45 days duration. Cardioversion can be expected within 30 minutes of administration. The major complication with this agent is the development of torsades de pointes, which can occur in up to 12.5% of patients, with 1.7% requiring cardioversion for sustained polymorphic ventricular tachycardia. These occur primarily within the first hour after administration. For this reason, patients given ibutilide are typically kept on monitor for several hours after administration. Procainamide is another intravenous agent that can be given to pharmacologically convert atrial flutter.
In general, controlling the ventricular rate in atrial flutter is more difficult than in atrial fibrillation. β-Blockers and calcium channel blockers are moderately effective in controlling the rate. Digoxin is less helpful since it only weakly blocks AV node conduction. Intravenous amiodarone, which has some β-blocking effect, has been shown to be at least as efficacious as digoxin.
The reentrant circuit in typical atrial flutter has been successfully mapped and includes an area of slow conduction called the isthmus, which is bound by the tricuspid annulus, the inferior vena cava, and the os of the coronary sinus. Ablation in the isthmus region interrupts the reentrant circuit and has been shown to be highly efficacious (90–100%) in permanently eliminating atrial flutter. In cost-effective analysis, ablation appears to be the preferred approach over cardioversion and pharmacologic prevention. Non–isthmus-dependent atypical atrial flutters can be more difficult to ablate. However, with current three-dimensional mapping systems (electroanatomic and noncontact high resolution), even these types of atrial flutter are being ablated with high success rates.
If attempts to ablate atrial flutter fail, the ventricular rate can be controlled by transcatheter ablation of the AV node or His bundle. With a long-standing, there may be a subsequent improvement in left ventricular function.
Atrial flutter is a recognized cause of peripheral embolization and stroke. The current recommendation is to treat patients with atrial flutter just as atrial fibrillation in terms of stroke prophylaxis. Many patients have bouts of atrial fibrillation even after successful atrial flutter ablation. Therefore, rigorous monitoring after atrial flutter ablation is recommended to determine which patients need long-term aspirin or anticoagulant therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree