35 Support Devices for High-Risk Percutaneous Coronary Interventions
 Introduction and Rationale
Introduction and Rationale
Complications of balloon angioplasty that threaten coronary blood flow, termed acute and threatened occlusion, usually require urgent surgical intervention and are the main causes of procedure-related morbidity and mortality. Before the advent of stents as a bail-out treatment for impending vessel closure, this complication occurred in about 6% of balloon angioplasty procedures. Patients who required emergent surgery in this setting had a 50% likelihood of suffering MI, with mortality rates as high as 10%.1 In these early studies, patient characteristics including compromised ventricular function, LMCA disease and multi-vessel disease, and older age were identified as risk factors for balloon angioplasty related mortality.2,3 With the development of coronary stents and advanced pharmacotherapy to seal dissections and improve blood flow in thrombotic lesions, respectively, the need for urgent surgery was reduced with a concomitant reduction in PCI-related morbidity and mortality.4
In the current era, studies have demonstrated that the need for urgent surgery after PCI has been reduced to less than 1% with a marked reduction in procedure-related mortality.5 In one comparison of patients treated in 1997–1998 with those treated in 1985–1986, the rate of in-hospital deaths, MI, and coronary artery bypass grafting (CABG) fell from 7.9% to 4.9%, despite the treatment of more complex lesions and stent use in only 71% of patients.6 Most of the difference between these periods was accounted for by the reduction in the need for emergent CABG from 3.7% to 0.4%.6 Nonetheless, morbidity and mortality among patients who required emergent CABG remained high.5 Moreover, the increased confidence afforded by stents and improved operator techniques and experience have prompted interventions on more complex lesions and patients with more severe cardiac and noncardiac diseases. In particular, results of the SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) trial now suggest reasonable outcomes in select patients with LMCA disease or multi-vessel coronary disease, while completed and ongoing trials of high-risk PCI with cardiac assist device placement indicate that extremely high-risk patient populations are, indeed, being increasingly considered for PCI.7,8 Given the large amount of the myocardium in jeopardy in these subsets, as well as the baseline comorbidities (including reduced ventricular reserve), the potential for severe clinical decompensation in such patients is a real concern. It has therefore become essential to precisely identify the predictors of risk and to consider the use of hemodynamic support for patients at high risk for procedural complications and in-hospital mortality. Although a number of investigators have used common sense definitions to classify patients at high risk, two studies systematically developed risk models which will be discussed in more detail.9–12 In the Mayo Clinic model, clinical and angiographic variables were used to predict in-hospital complications after PCI.8 The variables were age, shock, renal insufficiency, urgent procedures, heart failure, thrombus, and LMCA disease and multi-vessel disease. A score based on these factors predicted the risk of complications and identified a highest risk group with an event rate that exceeded 25%.12 In a similar study using 46,000 procedures in the New York State Hospital required PCI reporting system, investigators included nine factors in a risk score: (1) ejection fraction, (2) previous MI, (3) gender, (4) age, (5) hemodynamic state, (6) peripheral arterial disease (PAD), (7) CHF, (8) renal failure, and (9) LMCA disease. Using this risk score, a graded risk for in-hospital mortality was derived and validated. About 2% of all patients had a greater than 5% risk for in-hospital death, and about 4% of patients had a greater than 3% risk.11 Other studies have confirmed these risk factors for PCI-related complications.3,13,14 However, neither model may accurately represent the higher-risk patient populations undergoing intervention in the current era. In addition to these clinical risk factors, angiographic factors of lesion complexity (thrombus, calcification, bifurcations) have been shown to be associated with more dissections, distal embolization, and side-branch occlusions resulting in a threefold increase for in-hospital death.15
Several patient characteristics deserve separate discussion. Although female gender was initially associated with complications in early studies of balloon angioplasty, more recent studies have failed to demonstrate a very important effect on outcome.11,12,16,17 Similarly, patients with diabetes have more complex lesion characteristics and risk factors but no increase in in-hospital mortality after multivariable adjustment.18 More recently, baseline left ventricular dysfunction and extent of the myocardium in jeopardy during the procedure have re-emerged as perhaps the strongest clinical risk factors for intra-procedural decompensation and in-hospital mortality. Accordingly, recent studies on high-risk PCI have used the combination of severe ventricular dysfunction (represented by ejection fraction <30% to 35%) and either LMCA disease, last patent conduit, or multi-vessel disease as high-risk PCI inclusion criteria.19 Thus, it is clear that despite major advances in the technical and procedural performance of modern PCI, clinical and angiographic predictors of significant morbidity and mortality can be identified (Table 35-1). Moreover, it appears likely that increasing numbers of patients will be undergoing high-risk PCI, including the very old and those with LMCA disease, multi-vessel disease, and significant ventricular dysfunction. In many cases, bypass surgery is not a viable option, leaving PCI as the only remaining possible mechanism to improve ventricular function and reduce ischemic symptoms. Together, these data provide the rationale and impetus for the increased use of hemodynamic support during complex or high-risk PCI in the current era. The remainder of this chapter will discuss the approach to such patients, the devices that are currently available to provide support, and the results that may be achieved by using them.
TABLE 35-1 Predictors of Risk during Percutaneous Coronary Intervention
| Factor | Reference |
|---|---|
| Clinical and Patient-Related | |
| Older age | 3, 11, 12, 49 |
| Cardiogenic shock | 6, 11, 12, 49 |
| Recent myocardial infarction (MI) | 6, 11, 12, 49 |
| Congestive heart failure | 11–13, 49 |
| Prior coronary artery bypass grafting (CABG)/revascularization | 79 |
| Peripheral vascular disease | 11, 49 |
| Chronic renal insufficiency | 11–12, 49 |
| Angiographic | |
| Left main coronary artery/multi-vessel disease | 11, 12, 49, 80, 81 |
| Complex lesions (bifurcation, calcification, total occlusion) | 15 |
| Decreased thrombolysis in MI (TIMI) flow | 82 |
| Left ventricular dysfunction | 6, 11, 13, 14, 49 |
| Thrombus | 12, 15 |
 Approach to the Patient
Approach to the Patient
Mechanical circulatory support at the time of PCI has historically been instituted in one of two settings: (1) electively for presumed high-risk intervention and (2) emergently for peri-procedural hemodynamic instability. Specific indications, however, remain unclear because of limitations in performing large-scale, randomized trials and evaluating specific devices in individual patient subsets. Nevertheless, a review of existing literature and ongoing clinical trials provides a framework for patient selection and device selection when evaluating patients for elective or emergent support. Electively placed mechanical support is aimed at improving procedural success by minimizing myocardial ischemia and maintaining hemodynamic stability, thereby reducing clinical decompensation and resultant mortality in high-risk pre-selected patient subsets, as discussed in the previous section. In this setting, prophylactic insertion of the intra-aortic balloon pump (IABP) or the Impella ventricular assist device appear to have the most robust observational data for improving procedural success with a minimal increase in complications.19–25 Despite encouraging registry data, however, a recently completed randomized controlled trial failed to show clinical benefit to routine prophylactic IABP insertion in patients undergoing high-risk PCI.26 Whether the more powerful Impella device is able to provide benefit within the confines of a randomized controlled trial remains to be seen.8 The use of mechanical circulatory support in the emergent setting for patients with documented hemodynamic instability or cardiogenic shock is more familiar to interventional cardiologists. Instability may be present before PCI (as in acute MI with compromised ventricular function) or may develop as a consequence of procedural complications such as coronary dissection, poor coronary reflow, or thromboembolism. A common underlying finding in most of these patients is ventricular dysfunction. Although a large randomized clinical trial has not been performed, a pooled meta-analysis of IABP use in patients with acute ST elevation myocardial infarction (STEMI) found no benefit to IABP in conjunction with primary angioplasty, with some benefit seen in those receiving thrombolytic therapy.27 Available percutaneous ventricular assist devices appear to improve hemodynamic parameters in patients with acute clinical decompensation, but only registry data are available to suggest improved outcome.28 As a result, determining whether and which device to use in specific settings remains controversial and is primarily guided by experience. Device selection is based on several factors, including ease and rapidity of institution, level of invasiveness and complications, physician familiarity, requisite technical expertise, level of anticipated circulatory support, and available supportive clinical trial data. IABP is the least invasive and most familiar device and may be instituted rapidly, but it provides the least support, averaging 0.5 L/min augmentation in cardiac output.29 It may be left in place for several days, with a low vascular complication rate.30 Conversely, full CPS is significantly more invasive, requiring timely surgical and perfusionist collaboration for institution and removal, but can produce greater improvement in cardiac output approximating normal physiology. CPS cannot be maintained indefinitely, however, as hematologic and pulmonary complications increase as bypass time approaches 6 hours.30 Percutaneous ventricular assist devices (PVADs) such as the TandemHeart or the Impella provide an intermediate level of support approaching 2.5 to 4 L/min and can be placed emergently in the catheterization laboratory (cath lab) without surgical backup, which has prompted their increased use recently. Unlike the Impella, the TandemHeart requires trans-septal puncture to deliver the inflow cannula into the left atrium and requires a somewhat larger arterial cannula. Thus, only patients with larger femoral arterial diameter are able to accommodate device placement, which can be performed only by those skilled in the trans-septal technique. In both devices, the cannulae are larger than an IABP and may result in significant vascular morbidity. However, unlike full CPS, the use of PVAD has been successful for intermediate lengths of time, that is, up to 14 days. Relatively smaller cannulae, as with the Impella, are likely to reduce femoral complications. Historically, elective high-risk PCI has been performed safely with either provisional or prophylactic IABP support. As described above, however, preliminary randomized controlled trial data have suggested that routine prophylactic IABP support may offer little meaningful benefit in these patients.26 In those select patients who appear to require additional circulatory support, as defined by the inclusion criteria of completed and ongoing randomized trials, PVAD may be considered, with the caveat that the inherent increase in delay and invasiveness (particularly access site complications) may partially offset the benefit and that supportive randomized clinical trials still need to be completed.8 For patients who develop severe hemodynamic instability, cardiogenic shock, or frank arrest during PCI, bail-out use of the IABP appears beneficial and is certainly the most familiar and rapid strategy. Cath labs experienced in rapid PVAD placement may opt for these larger, more powerful devices. Developing data on hemodynamic parameters that might predict meaningful recovery, such as cardiac power output, would at least theoretically support their use.31 Although full CPS may be considered in catheterization laboratories equipped and staffed for timely initiation, its clinical use has now been relegated to anecdotal experience. Thus, in emergent settings, PVADs appear more promising but require specialized technical expertise.
 Description of Devices
Description of Devices
Intra-aortic Balloon Pump
The IABP was first used clinically in cardiogenic shock by Kantrowitz in 1968.32 As its application expanded to include refractory angina, severe hemodynamic compromise, and postcardiotomy pump failure, and with the advent of percutaneous insertion, the IABP was one of the first hemodynamic devices used to support high-risk PCIs.32–34
The rapid filling of the balloon in early diastole augments diastolic pressure and thus leads to increased coronary perfusion pressure, whereas deflation of the balloon at end-diastole reduces effective aortic volume and decreases aortic systolic pressure leading to lower left ventricular afterload. The net effect is a decrease in myocardial oxygen requirements from lower systolic wall tension and an increase in coronary perfusion pressure, improving the myocardial supply-and-demand balance. Cardiac output increases because of the improved myocardial contractility as a result of the increased coronary blood flow and the reduced afterload.35,36
Clinical Trials
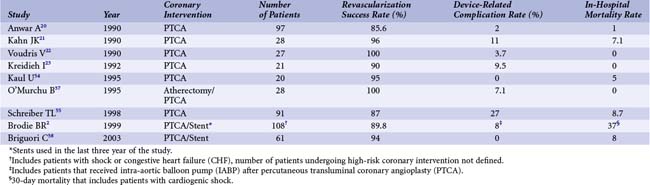
Much of the data on the use of IABP as well as other circulatory support devices during high-risk PCI comes from the pre-stent era of coronary interventions. Voudris et al showed that support with IABP during elective high-risk angioplasty is both safe and feasible.22 During a 13-month period (in 1987–1988), 27 patients considered high risk because of decreased left ventricular function or multi-vessel disease underwent angioplasty with IABP support. Primary success, according to contemporary American College of Cardiology (ACC) guidelines, was achieved in all 27 patients. There were no major cardiac events during hospitalization, and there was only one IABP-related vascular complication. After a mean follow-up period of 13 months, there were 2 deaths, 1 cardiac transplantation, and 6 cases of symptom-driven target vessel revascularization (TVR, 22% rate of recurrent angina), which was also successfully performed with IABP support.22 Similar outcomes were reported by Kahn et al in a group of 28 high-risk patients during the same period.21 The most common high-risk feature in this cohort of patients was severe left ventricular dysfunction, but some patients had critical stenoses in the left main coronary artery (LMCA) or a single remaining coronary artery. Procedural success was 96% (90 of 94 lesions were successfully dilated). There were 11 cases of intra-procedural hypotension, although the augmented diastolic pressure was maintained over 90 mm Hg in all cases and angioplasty was completed in all patients. Vascular complications associated with the IABP occurred in 11% of the patients. In another series of 21 patients with similar high-risk features, 90% of lesions attempted were successfully dilated without hemodynamic compromise.23 Device-related complications (hematoma) occurred in 10% of the cases, and procedure-related complications occurred in 14% of the cases.23 The beneficial effects of IABP support were also shown in high-risk coronary rotational atherectomy.37 In this retrospective analysis, 28 patients scheduled to undergo rotational atherectomy were placed on IABP support before the coronary intervention. This group was compared with 131 patients with high-risk coronary lesions who did not have an IABP placed a priori. The group that received a planned IABP comprised patients who were older, had more left ventricular dysfunction, and had a higher incidence of multi-vessel disease. While systolic hypotension occurred in 11% of the patients in the study group, diastolic pressure augmentation provided by the IABP allowed successful completion of the procedure in all patients. Hypotension necessitating IABP placement occurred in 7% of the patients in the comparison group. Slow flow occurred at a similar rate in both groups; however, 27% of the patients in the comparison group who experienced slow flow developed a non–Q wave MI compared with none in the study group. There were no differences in the rate of transfusion requirements or vascular complications.37 In a more recent study by Brodie et al, IABP was shown to reduce peri-procedural events.2 The group in this study consisted of 213 patients who presented with acute MI and received an IABP. In contrast to the earlier studies discussed, the majority of patients in this study were treated with stents, and about a third were treated with abciximab, reflecting more contemporary treatments. While the indication for the IABP in most cases was cardiogenic shock, there were 80 patients who were hemodynamically stable but were considered high risk because of left ventricular dysfunction. In this group, the use of IABP support led to a decreased incidence of prolonged hypotension, cardiac arrest, and ventricular fibrillation; however, the difference was not statistically significant because of the low number of patients in this group. IABP use was associated with an increased risk of major bleeding and higher transfusion rates. While IABP use during high-risk coronary interventions has been shown, at least in registry and case series data, to be effective in supporting the circulation, the increased rates of vascular and hemorrhagic complications associated with its use demands careful patient selection.2,21,22,24,37 For this reason, a strategy of provisional IABP support was compared with prophylactic placement of IABP in high-risk interventions in a retrospective, non-randomized study.38 Sixty-one patients who received elective IABP were compared with 72 patients in whom support was initiated only when clinically necessary. The patients in the elective IABP group were slightly older, but other high-risk features were similar, including severity of left ventricular dysfunction (ejection fraction [EF] <30%) and rates of multi-vessel disease and unstable angina. Rates of stent and glycoprotein inhibitor use were similar between the two groups. The rates of slow flow were similar in both groups, but hemodynamic deterioration occurred only in patients (15%) in the provisional IABP group, and all received urgent IABP support. Rates of vascular complications were low in this study, with only two patients in the provisional IABP group developing groin hematomas. There were no cases of major bleeding. Although not statistically significant, three deaths occurred in the provisional IABP group in patients who required urgent placement of an IABP compared with one death in the elective group.38 Most recently, however, routine prophylactic use of IABP in high-risk PCI has come into question, following the preliminary results of a large randomized trial.26 These findings, if confirmed, highlight the need for randomized controlled trial data to fully elucidate the proper role of any cardiac assist device in high-risk PCI. A summary of the published observational trials using IABP during high-risk PCI is shown in Table 35-2.