Chapter 63
Superior Vena Cava Obstruction
Surgical Treatment
Peter Gloviczki, Manju Kalra
Symptoms of venous congestion of the head and neck secondary to occlusion of the superior vena cava (SVC) or innominate veins develop in about 15,000 patients each year in the United States.1 SVC syndrome is caused by malignant tumors of the lung and mediastinum in 60% of cases.2 The most frequent nonmalignant causes include placement of intravenous catheters or pacemaker wires and mediastinal fibrosis. Surgical treatment of SVC syndrome in patients with advanced malignant disease is frequently palliative, but it is usually curative for those with benign disease. Few areas of venous disease provide a more satisfying experience for both the patient and the vascular specialist than reconstruction for SVC syndrome. Relief from severe, frequently incapacitating symptoms of venous congestion of the head and neck is almost instantaneous, and the benefit after reconstruction is generally long lasting. Endovascular treatment of both acute and chronic SVC occlusion has become the first line of treatment for most patients and is discussed in detail in Chapter 64. In this chapter, we review the etiology, clinical findings, and diagnostic evaluation of SVC syndrome and present techniques and results of open surgical treatment of SVC occlusion.
Etiology
The first case report of an SVC obstruction, described by William Hunter in 1757, was due to an aortic aneurysm.3 Aortic aneurysms remained the second most common cause of SVC syndrome after primary malignant thoracic tumors until the mid-1900s.2 The incidence of tuberculous and syphilitic mediastinitis decreased markedly early in the 20th century. Lung cancer with mediastinal lymphadenopathy and primary mediastinal malignant tumors have become the most frequent causes of malignant SVC syndrome in the past 3 decades.1–7 Primary mediastinal malignant tumors leading to SVC syndrome include mediastinal lymphoma, medullary or follicular carcinoma of the thyroid, thymoma, teratoma, angiosarcoma, and synovial cell carcinoma.1–8 Of malignant SVC syndromes, non–small cell lung cancer is the cause in 50%, followed by small cell lung cancer (22%), lymphoma (12%), metastatic cancer (9%), germ cell cancer (3%), and thymoma (2%).1 Benign disease is the cause of SVC syndrome in 40% of cases. Mediastinal fibrosis and granulomatous fungal disease, such as histoplasmosis, have been the most frequent benign causes of SVC and innominate vein obstruction.7,9–13
However, the rapid increase in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades has resulted in greater numbers of patients with SVC obstruction of benign etiology. Millions of central venous catheters and 397,000 pacemakers are now implanted annually in the United States and are associated with upper extremity or central vein deep venous thrombosis in 7% to 33% of patients.14,15 SVC syndrome reportedly occurs in 1% to 3% of patients with central venous catheters and in 0.2% to 3.3% of patients with implanted pacemakers.2 Previous radiotherapy to the mediastinum, retrosternal goiter, and aortic dissection can also cause SVC syndrome. The risk for venous thrombosis is increased in patients with thrombophilia, such as the factor V Leiden mutation, and in those with deficiencies of circulating natural anticoagulants, such as antithrombin III, protein S, and protein C.
Clinical Findings
Signs and symptoms of venous congestion of the head, neck, and upper extremities are determined by the duration and extent of the occlusive venous disease and by the amount of collateral venous circulation that develops. Patients with SVC syndrome have a feeling of fullness in the head and neck that is exacerbated when bending over or lying flat in bed. The severity of the disease can easily be graded by the number of pillows needed by the patient to sleep comfortably. Venous hypertension may cause dyspnea on exertion or orthopnea, headache, dizziness, syncope, or visual symptoms (Table 63-1).1,16–18 Patients may complain of mental confusion or coughing. Dilated neck veins and swelling of the face, neck, and eyelids are the characteristic physical signs most commonly seen (Fig. 63-1). Prominent chest wall collateral veins are frequently present, as are ecchymosis and cyanosis of the face. Although symptoms are usually localized to the head and neck, mild to moderate upper extremity swelling may also develop. Additional signs and symptoms of malignant SVC syndrome include hemoptysis, hoarseness, dysphagia, weight loss, lethargy, and palpable cervical tumor or lymph nodes. Patients with lymphoma may also complain of fever and night sweats.
Table 63-1
Signs and Symptoms of Superior Vena Cava Syndrome of Benign Etiology in 70 Patients
| Signs and Symptoms | Number of Patients (%) |
| Feeling of fullness in the head or neck | 61 (87) |
| Dyspnea on exertion or orthopnea | 39 (56) |
| Headache | 27 (39) |
| Dizziness or syncope | 25 (36) |
| Visual problems | 11 (16) |
| Cough | 10 (14) |
| Nocturnal oxygen requirement | 3 (4) |
| Protein-losing enteropathy | 1 (1) |
| Head and neck swelling | 65 (93) |
| Large chest wall venous collaterals | 40 (57) |
| Facial cyanosis | 24 (34) |
| Arm swelling | 23 (33) |
| Pleural effusion | 2 (3) |
From Rizvi AZ, et al: Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg 47:372, 2008.
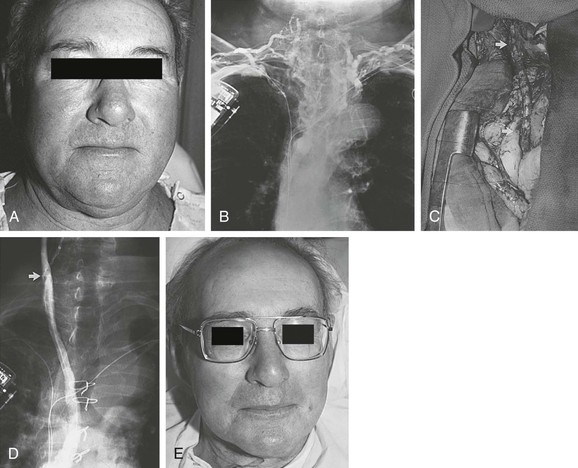
Figure 63-1 A, Severe symptomatic superior vena cava (SVC) syndrome in a 69-year-old man. B, A bilateral upper extremity venogram confirms thrombosis of the SVC and both innominate veins after placement of pacemaker lines bilaterally. C, Right internal jugular vein/right atrial appendage spiral saphenous vein graft (SSVG). Arrows indicate anastomoses. D, Postoperative venogram confirming graft patency. E, Photograph of the patient 5 days after SSVG placement. The clinical result is excellent 8 years after the operation. (From Gloviczki P, et al: Superior vena cava syndrome: endovascular and direct surgical treatment. In Gloviczki P, Yao YST, editors: Handbook of venous disorders, London, 1996, Chapman & Hall, pp 580-599.)
Diagnostic Evaluation
The diagnosis of SVC obstruction is usually suggested from a detailed clinical history and physical examination. The clinical diagnosis may be confirmed by a variety of tools, including plain film radiography, ultrasonography, computed tomography (CT), venography, and magnetic resonance imaging. The appropriate diagnostic study for an individual patient will demonstrate not only the underlying cause but also the site and extent of obstruction as well as the routes of collateral venous circulation.
Radiography
Plain film radiographs of the chest are readily available and are often abnormal in patients with SVC obstruction. Findings most commonly include mediastinal widening, right hilar mass, pleural effusion, bilateral diffuse infiltrates, and upper lobe collapse; however, a normal radiograph of the chest does not preclude the diagnosis of SVC obstruction.1,5,16 On occasion, dilated collateral veins may be visible, especially enlargement of the azygos vein or superior intercostal vein (aortic nipple) draining the hemiazygos system. In more than 90% of patients, a diagnosis of SVC syndrome can be made on the basis of the clinical findings and plain chest radiograph.16
Ultrasonography
Ultrasound evaluation with duplex scanning (see Chapter 18) is an effective, noninvasive screening technique for patients with suspected SVC obstruction. Although direct visualization of the SVC is not possible with transthoracic duplex ultrasound, valuable information can be obtained. The subclavian and internal jugular veins are accessible to sonographic evaluation and can provide indirect evidence of SVC patency or obstruction. In the presence of SVC obstruction, the normal variation in respiratory flow caused by changes in intrathoracic pressure seen in patent subclavian veins is lost. This can easily be demonstrated by reduced or no change in the diameter and blood flow through the subclavian veins in response to respiratory maneuvers, such as a sudden sniff or a Valsalva maneuver. Collateral vessels can be detected within the chest wall or in the mediastinum.
Computed Tomographic Angiography
CT accurately depicts the location and extent of the obstruction and also distinguishes various types of benign and malignant mediastinal disease.19–23 Any mass or tumor is easily identified, and central lines or pacemaker wires are well seen. Injection of intravenous contrast material delineates the central veins, and three-dimensional reconstruction helps map the venous occlusion. CT will also identify the collateral pathways, including (1) the azygos-hemiazygos pathway, (2) the internal mammary pathway, (3) the lateral thoracic–thoracoepigastric pathway, and (4) the vertebral pathway and small mediastinal veins. Less commonly, unusual shunts, including hepatic parenchyma as an intense focal enhancement in the medial segment of the left lobe of the liver, and pulmonary pathways are identified on CT.22,23 No definite relationship between the level of occlusion and the number of collateral pathways has been identified.
Contrast-Enhanced Venography
Venography has been considered the “gold standard” for accurate depiction of central venous obstruction and is used to provide an anatomic roadmap before reconstructive surgery. Venography also depicts the presence and direction of venous collateral flow. It is performed by simultaneous injection of contrast material in bilateral superficial arm veins. Stanford and Doty described four venographic patterns of SVC syndrome, each having a different venous collateral network, depending on the site and extent of SVC obstruction24 (Fig. 63-2 and Table 63-2). Type I is partial and type II is complete or nearly complete SVC obstruction, with flow in the azygos vein remaining antegrade. Type III is 90% to 100% obstruction of the SVC with reversed azygos blood flow (Fig. 63-3A), whereas type IV is extensive mediastinal central venous occlusion with venous return occurring through the inferior vena cava (see Fig. 63-1B).
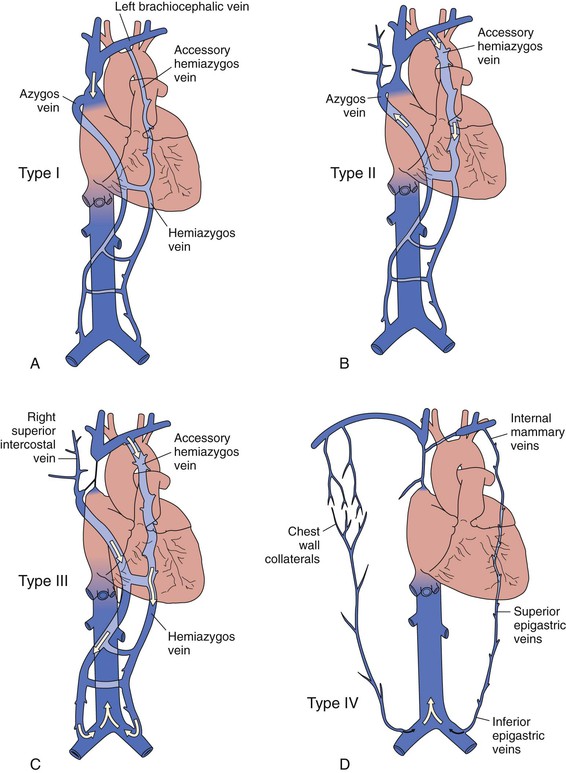
Figure 63-2 Venographic classification of superior vena cava (SVC) syndrome according to Stanford and Doty.24 A, Type I: high-grade SVC stenosis but still normal direction of blood flow through the SVC and azygos vein. There is increased collateral circulation through the hemiazygos and accessory hemiazygos veins in type I. B, Type II: greater than 90% stenosis or occlusion of the SVC but a patent azygos vein with normal direction of blood flow. C, Type III: occlusion of the SVC with retrograde flow in both the azygos and hemiazygos veins. D, Type IV: extensive occlusion of the SVC and innominate and azygos veins with chest wall and epigastric venous collaterals. (Redrawn from Alimi YS, et al: Reconstruction of the superior vena cava: the benefits of postoperative surveillance and secondary endovascular interventions. J Vasc Surg 27:298-299, 1998.)
Table 63-2
Venographic Classification* in 70 Patients Who Underwent Reconstruction for Benign Superior Vena Cava Syndrome
| Type | Description | Number of Patients (%) |
| I | Stenosis (up to 90%) of the SVC with patency and antegrade flow of the azygos–right atrial pathway | 6 (9) |
| II | >90% stenosis or occlusion of the SVC with patency and antegrade flow in the azygos–right atrial pathway | 16 (23) |
| III | >90% stenosis or occlusion of the SVC with reversal of azygos blood flow | 28 (40) |
| IV | Occlusion of the SVC and one or more of the major caval tributaries, including the azygos systems | 20 (28) |
| Total | 70 (100) |
SVC, Superior vena cava.
From Rizvi AZ, et al: Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg 47:372, 2008.
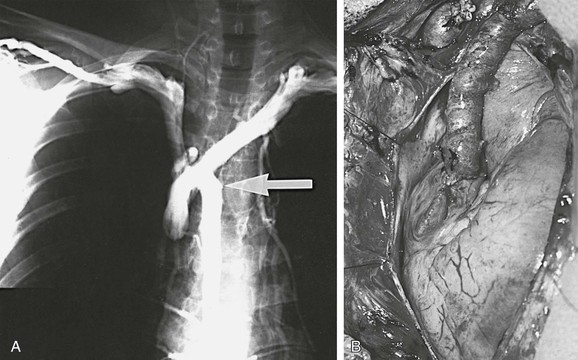
Figure 63-3 A, Bilateral upper extremity venogram documenting obstruction of the superior vena cava and retrograde flow through the azygos vein (arrow). B, Left innominate vein/right atrial appendage spiral saphenous vein graft. (From Gloviczki P, et al: Reconstruction of the vena cava and of its primary tributaries: a preliminary report. J Vasc Surg 11:373-381, 1990.)
During upper extremity venography, only veins and collateral pathways between the injection site and the right atrium are visualized. The internal jugular veins, frequently used for inflow for surgical bypass, are not visualized.
Magnetic Resonance Venography
Advantages of magnetic resonance venography (MRV) include the ability to demonstrate anatomic structures in multiple planes and to delineate the central venous chest circulation and collateral vessels. MRV is a relatively noninvasive modality and does not require the administration of iodinated contrast material. A disadvantage is its contraindication in patients with pacemakers and aneurysm clips. Recent problems with use of gadolinium in renal insufficiency have significantly decreased the utility of MRV in this group of patients.
Conservative Therapy
Conservative measures are used first in every patient to relieve symptoms of venous congestion and to decrease the progression of venous thrombosis. Such measures include elevation of the head on pillows during the night, modification of daily activities by avoiding bending over, and avoidance of wearing constricting garments or a tight collar. Patients frequently need diuretic agents to decrease, at least temporarily, excessive edema of the neck and head.
Patients with acute SVC syndrome caused by malignant disease are generally treated with intravenous unfractionated or low-molecular-weight heparin, followed by warfarin, to prevent recurrence and to protect the venous collateral circulation. Thrombolytic treatment is considered in most patients with benign acute SVC syndrome, whereas those with metastatic malignant disease are candidates for treatment with endovascular stents, with or without thrombolytic therapy25,26 (see Chapter 64).
Symptoms of SVC syndrome caused by mediastinal malignant tumors frequently improve after irradiation, chemotherapy, or combination chemoradiotherapy based on tumor histology.1 Symptoms resolve in 80% of patients within 4 weeks.
In the past 2 decades, endovascular treatment with stents has become the first-line therapy for both malignant and benign SCV syndrome. For technique and results of endovenous therapy, readers are referred to Chapter 64.
Surgical Treatment
Indications
Patients with SVC syndrome can have severe, frequently incapacitating symptoms that cannot be alleviated by conservative measures. Patients with benign disease who have extensive chronic venous thrombosis (type III or IV) not anatomically suitable for endovascular treatment and those with less extensive disease (type I or II) who have failed to respond to endovascular therapy are candidates for open surgical reconstruction. There are multiple, frequently just small series in the literature reporting successful surgical reconstruction for a variety of benign causes of SVC syndrome, including granulomatous and idiopathic mediastinal fibrosis, central venous catheters, pacemaker electrodes, and ventriculoatrial shunts, as well as antithrombin III deficiency and idiopathic venous thrombosis.9–11,17,18,27–29
Surgical reconstruction of the SVC has also been performed in patients with different types and stages of malignant disease.30–41 Patients with a malignant tumor should undergo reconstruction through a median sternotomy only if their life expectancy is more than 1 year. Extra-anatomic subcutaneous bypass between the jugular vein and the femoral vein with a composite saphenous vein graft or polytetrafluoroethylene graft is an alternative if symptoms are severe and endovascular techniques fail or are not possible.42–45
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


