Superior Mesenteric Artery Aneurysm
MATTHEW R. SMEDS and GUILLERMO A. ESCOBAR
Presentation
A 73-year-old man had a repair of a strangulated inguinal hernia and small bowel resection, complicated by a left ureteral injury and subsequent urinoma. A CT scan obtained 10 days after the index procedure revealed a 3-cm aneurysm of the mid–superior mesenteric artery (SMA) surrounded by loculated fluid collections (Fig. 1). The fluid collections are consistent with an infected urinoma, notable for septations, mesenteric stranding, and dilated bowel.
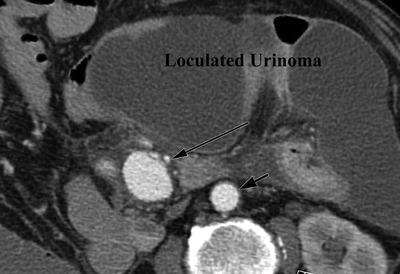
FIGURE 1 Axial CTA images demonstrating a large, inflammatory rind surrounding an urinoma and SMA aneurysm. The long arrow indicates the SMA and a 3-cm SMA aneurysm. Short arrow indicates the aorta.
Differential Diagnosis and Epidemiology
Visceral artery aneurysms are rare clinical entities that are identified in less than 0.2% of autopsy studies. These aneurysms may be true aneurysms, mycotic, aneurysmal degeneration of dissections, or pseudoaneurysms. They are most commonly found in the splenic or hepatic distribution. However, aneurysms of the SMA and its branches are uncommon and represent only 6% to 7% of all visceral artery aneurysms. True, spontaneous SMA aneurysms are usually located in the proximal artery but can be located anywhere in the mesentery and sometimes appear as multiple aneurysms scattered in the mesentery.
Visceral artery aneurysms as a whole have the following etiologies: atherosclerotic (68%), mycotic (13%), traumatic (10%), collagen disorders (6%), and giant cell arteritis (3%). Meanwhile, SMA aneurysms are mycotic in almost two thirds of reported cases (usually endocarditis in younger patients or in concert with a severe intra-abdominal infection). Atherosclerotic degeneration accounts for the second most common cause of SMA aneurysms, while it is the most common etiology in older patients. SMA aneurysms can also follow primary dissections, or as a consequence of aortic dissections. Other causes include genetic disorders (Marfan’s syndrome, Ehlers-Danlos syndrome, neurofibromatosis type I, Klippel-Trenaunay syndrome etc.), iatrogenic pseudoaneurysms, and inflammation from autoimmune disorders (giant cell arteritis).
Up to a third of visceral aneurysms may be associated with a nonsplanchnic aneurysm elsewhere in the body that usually is spontaneous, or they may be linked to arterial dissections. Historic reports of splanchnic artery aneurysm ruptures had high mortality rates between 20% and 70% (especially when related to pregnancy); however, recent reviews suggest that the modern mortality ranges between 15% and 20%.
Clinical Presentation of SMA Aneurysms
SMA aneurysms are usually asymptomatic and incidentally found on CT scans. Patients may experience vague abdominal pain, nausea, and vomiting––presumably secondary to distal embolization, perianeurysm inflammation, or compression. They may also present with various degrees of gastrointestinal hemorrhage secondary to fistulizing into bowel. Spontaneous intra-abdominal rupture of the aneurysm or acute SMA thrombosis can present with a more dramatic clinical presentation due to bleeding or acute mesenteric ischemia, respectively. Rupture rates are ill defined but estimated at approximately 10%. Bleeding may occur into the retroperitoneum or free rupture into the peritoneal cavity, the latter being linked to most fatalities. On physical exam, patients may have a palpable pulsatile mass or an audible bruit in the epigastrium. In patients presenting with abdominal pain alone, other causes of abdominal pain need to be ruled out first unless there is strong evidence of embolic injury to the bowel.
This case suggests the possibility of a mycotic etiology of the SMA aneurysm due to the associated infection and rather remote location from the origin of the artery. However, due to the patient’s older age, it is possible to be from the more benign, spontaneous/atherosclerotic etiology. If so, then elective repair may be considered once his acute illness resolves. However, due to the real concern for a mycotic aneurysm, close surveillance with repeated short interval imaging should be obtained prior to dismissing the possibilities for urgent repair.
Case Continued
The patient was treated with intravenous antibiotics and underwent percutaneous drainage of the urinomas and had a percutaneous nephrostomy tube placed. Two weeks later, a CT angiography (CTA) demonstrated an increase in size of the aneurysm to 4.5 cm (Fig. 2), and there is new stranding in the mesentery around the SMA without evidence of rupture. The celiac, superior mesenteric, and inferior mesenteric arteries are all grossly within normal limits. He denies abdominal or back pain, and there is no blood in his stool. On examination, his vital signs are normal. He has an open abdominal skin incision with good granulation tissue at the base, and the left nephrostomy tube has clear urine within. There are no pulsatile abdominal masses and has normal pulses in his extremities. Prior to this recent illness, he was in otherwise good health and was physically quite active for his age.
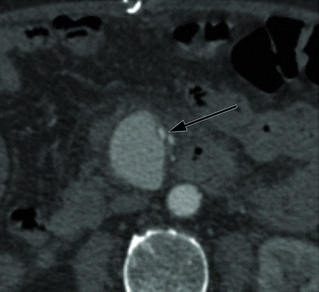
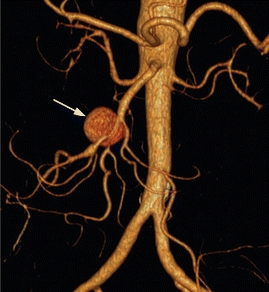
FIGURE 2 Left-sided image demonstrates axial CTA images of the same patient, 2 weeks after draining the fluid collections. Arrow identifies the SMA aneurysm surrounded by inflammation, which has grown to 4.5 cm. Right-sided image is a 3-D reconstruction of the abdominal aorta and its branches. Arrow indicates the SMA aneurysm.
Discussion
The rapid interval growth and perimesenteric fat stranding are very concerning for a mycotic SMA aneurysm, as well as for impending rupture. Therefore, surgical planning is to follow. Due to the infectious nature, either simple ligation or an autologous conduit (vein) should be chosen. Saphenous vein is the conduit of choice, and therefore, he should undergo preoperative vein mapping of the veins to select the ideal conduit.
Diagnosis, Natural History, and Treatment
Diagnosis of SMA aneurysms may be done via CT or MR angiography, conventional digital subtraction angiography, or duplex imaging in thin patients. Sonographic examination will demonstrate a hypoechoic pulsatile mass. Duplex is most helpful for screening or surveillance of known aneurysms, while angiography may be more useful for operative planning. High-resolution CT or MR angiography can determine the location of the aneurysm in relation to branch vessels, the degree of collateralization, and the presence of coexisting diseases. Three-dimensional reconstructions and straight line reconstruction software can be very helpful for endovascular case planning. Success at symptom management, aneurysm exclusion, and patency after open reconstructions is about 90% at 4 years, and there is a negligible mortality in elective procedures done in experienced centers, and about 15% overall.
Despite first being described in 1812, the natural history of SMA aneurysms remains unknown due to its scarcity and publication bias. Thus, the timing of SMA and visceral artery aneurysm repair is not well established, and their growth and rupture rates may be overestimated. As an example, while gastroduodenal artery aneurysms are associated with celiac artery occlusions and stenosis, they are reported to grow in only about 38% over 2 years, while the rest remain stable. Fear of rupture and embolization from aneurysms greater than 2 cm drive operative management. Patients with symptomatic aneurysms or those larger than 2 cm should be considered for repair. Aneurysms found close to the wall of the intestine are managed with a limited segmental bowel resection including the affected mesentery. Multiple aneurysms scattered in the bowel cannot be managed in toto and therefore require watchful waiting and only intervening in symptomatic cases.
Smaller aneurysms, mesenteric dissections, and those felt to be too high risk for repair may be observed with serial ultrasound (when possible) or CT angiogram and generally have a rather slow growth rate. In patients with acute mesenteric dissections, anticoagulation for a few months, followed by antiplatelet agents, may be initially considered to decrease the risk of embolization and bowel ischemia, but these usually do not become aneurysmal after many years of observation.
Open Surgical Approach—Technical Tips and Outcomes
Surgical repair of SMA aneurysms can surprisingly be performed often via simple ligation (about 30% of reported cases), especially in cases of acute hemorrhage. The presence of collateral vessels will determine if simple ligation will suffice, and this can be further verified by temporary occlusion of the SMA segment to be ligated with assessment of bowel viability intraoperatively. Aneurysmorrhaphy is best done in primarily saccular aneurysms, where the top of the aneurysm can be removed and a linear repair can be done. If formal arterial reconstruction is needed, the greater saphenous vein is the conduit of choice in cases of infection or when a concomitant bowel resection is needed. In the absence of these conditions, a synthetic graft may be considered to replace or patch the artery.
Open surgical repair of SMA aneurysms is performed under general anesthesia in patients who are deemed acceptable surgical risk. A vertical midline abdominal incision is generally made (or transverse), and the omentum and transverse colon are retracted superiorly, with the small bowel and mesentery retracted caudally. If the aneurysm is in the proximal SMA, this will be retroperitoneal between the aorta and pancreas and posterior to the superior mesenteric vein and will require an approach via left-sided visceral rotation. Meanwhile, aneurysms that lie further out than the pancreas will require an anterior approach through the mesocolon-small bowel mesentery junction. Some aneurysms may require both types of exposures to obtain proximal vascular control (via the retroperitoneum) and then vascular reconstruction/repair from the anterior approach.
The SMA is located anteriorly within the mesentery, at the junction of the small bowel mesentery and transverse mesocolon where it crosses over the duodenum. Proximal and distal control is obtained while avoiding the superior mesenteric vein, which normally lays to the right of the artery. The further from the SMA origin, greater care is needed to isolate and not injury branches that emerge from the lateral and posterior surfaces of the SMA. This is especially more challenging the more distal the aneurysm lies from the SMA origin where branches that are not seen are either avulsed during the dissection or will bleed continuously when entering the aneurysm sac during repair. Temporary occlusion of the SMA proximal and distal to the aneurysm, followed by an assessment of bowel viability, can allow for determination of the success of simple ligation. If aneurysmorrhaphy or bypass is planned, the patient is systemically heparinized, the vessels are clamped and the branches arising from the aneurysm sac are either temporarily occluded (for aneurysmorrhaphy or patching) or ligated (for SMA interposition grafting/bypass). The sac is opened and contents evacuated. Redundant wall is resected, and the SMA is reconstructed, or closed primarily (with or without a patch).
A more proximal aneurysm may require a retroperitoneal approach from the left side, with right medial reflection of the colon, pancreas, and spleen. Bypass arising from the aorta to a normal, distal SMA with ligation of the aneurysm is another option for very long or proximal aneurysms. After restoration of flow, the bowel should be investigated closely for viability. The mesentery is closed over the repair, heparin is reversed, and the abdomen is closed in the standard fashion.
Endovascular Approach—Technical Tips and Outcomes
Endovascular therapy provides an evolving option for patients in whom an open surgical approach may be suboptimal, but mostly limited to noninfectious etiologies. These alternatives include coil embolization of the aneurysm or feeding vessels and/or placement of a covered stent across the aneurysm and potentially the use of “flow-diverting” stents to theoretically allow flow into the branches, while promoting thrombosis of the aneurysm sac. As all these techniques can significantly impair intestinal blood flow, preprocedure evaluation of the small bowel branch vessels using 3-D CTA or a high-magnification angiogram is critical. Without adequate collateralization, bowel ischemia may result after endovascular exclusion of the blood flow.
Access is usually obtained via the left brachial or axial artery due to the acuity of the SMA angle off the aorta, and long or stiff stent grafts may not maneuver from the aorta into the SMA. Once the vessel is selected, a long sheath is advanced into the artery, and the covered stent or coils are delivered. Use of stent grafts requires adequate proximal and distal landing zones to ensure graft stability. There are no long-term data available to determine longevity of these repairs, but multiple case series describe their utility (mostly in emergent cases). “Flow-diversion” has been limited to about 28 cases reported using the Cardiatis Multilayer Stent as a possible alternative to traditional open and endovascular approaches.
Operative Technique and Findings
After lysis of adhesions, the aneurysm is palpated at the base of the thickened mesentery. The lesser sac is entered, and the pancreas is elevated, allowing identification of the proximal SMA emerging beneath the uncinate process that is encircled with a vessel loop. The mesentery is pulled inferiorly, and the dissection is continued along the SMA until the aneurysm is encountered approximately 3 to 4 cm from the origin. The SMA distal to the aneurysm is identified and encircled with vessel loops. Five branches emanating from the aneurysm are also individually encircled with vessel loops. A length of greater saphenous vein is harvested from the left leg. The patient is systemically heparinized to a goal activated clotting time greater than 250 seconds, and the SMA is clamped proximally and distally. The aneurysm is opened and reveals that the back wall appears to have blown out. The five branches all have excellent back bleeding, and four are ligated. A segment of dilated bowel appears cyanotic, so the decision is made to reconstruct the artery. The saphenous vein is reversed and sewn as an interposition graft in an end-to-end fashion between the normal proximal and distal SMA. Flow is restored, and the bowel is inspected. There are good pulses and Doppler signals noted throughout the mesentery, and the bowel is now pink. The mesentery is closed over the vein graft, and the abdomen is irrigated with copious warmed saline. The abdominal incision is closed with the skin left open, and the leg incision is closed in multiple layers.
Postoperative Management
Postoperatively, the acuity of monitoring is dependent on the patient’s preoperative clinical status and medical comorbidities. All patients should be monitored closely for bowel ischemia, which may be identified by progressive abdominal pain, bloody bowel movements, persistent nausea, vomiting or diarrhea, and hemodynamic instability. Laboratory studies associated to bowel ischemia are also obtained and may include liver function tests, as well as white blood cell counts and serum lactate levels. There should be a low threshold for return to the operating room for a second look if bowel ischemia is entertained.
Patients treated via an endovascular approach will typically have a lower perioperative morbidity and faster recovery. Access sites should be monitored for hematoma development, especially when the brachial artery was used. Endovascular techniques may result in “new” complications, including endoleak resulting from patent collateral vessels, and as such, long-term CTA monitoring of these patients may be necessary and conversion to open may be needed (Table 1).
TABLE 1. SMA Aneurysm Repair




