69 Sudden Cardiac Death in Athletes
Causes of Sudden Cardiac Death in Athletes
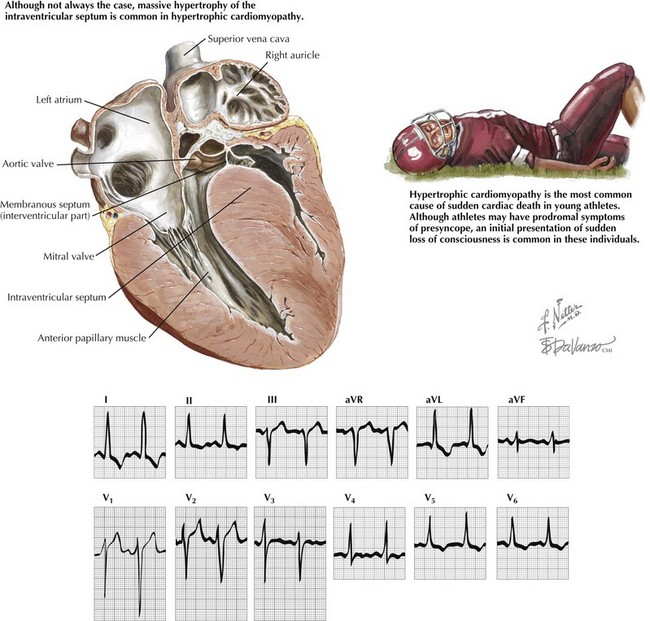
Hypertrophic Cardiomyopathy
Ventricular tachycardia and ventricular fibrillation are the most frequent etiologies of sudden cardiac death in patients with HCM. The six features associated with greatest risk for sudden cardiac death are prior cardiac arrest or sustained ventricular tachycardia, family history of one or more premature HCM-related deaths, syncope, hypotensive blood pressure response to exercise, multiple or prolonged episodes of nonsustained ventricular tachycardia on ambulatory monitoring, and left ventricular (LV) wall thickening greater than 30 mm (Fig. 69-1).
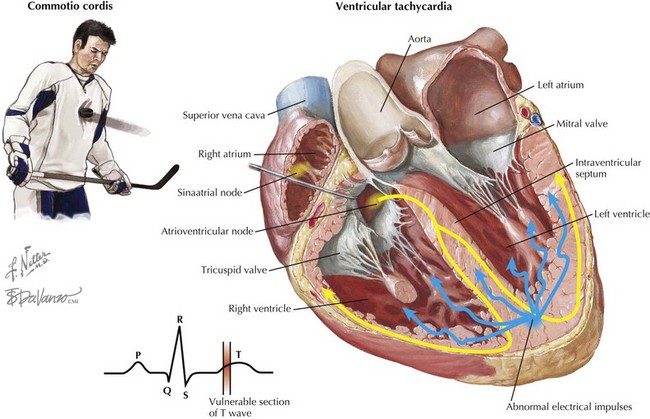
Commotio Cordis
Commotio cordis refers to a blunt, nonpenetrating blow to the chest wall that results in sudden cardiac death. Based on experimental data, it is thought that commotio cordis primarily occurs only when chest trauma occurs just before the peak of the T wave during repolarization. In a swine model of commotio cordis, it was demonstrated that the vulnerable point in the cardiac cycle was between 15 and 30 ms before the peak of the T wave. In this model, 90% of the chest blows (either with a ball or a wooden bat) during this portion of the cardiac cycle induced ventricular fibrillation. Blows to the chest outside of this time period did not induce ventricular fibrillation but did produce brief episodes of polymorphic ventricular tachycardia. In settings of electrolyte abnormalities or underlying cardiac disease, the induction of polymorphic ventricular tachycardia may also result in ventricular fibrillation and sudden cardiac death. Complete heart block, ST-segment elevation, and left bundle branch block were observed when impacts to the chest occurred during the QRS complex and not during the vulnerable phase. Complete heart block was only observed with chest blows during the QRS complex and not with impacts at other times during the cardiac cycle. Coronary angiography performed after blunt injury did not reveal significant coronary artery abnormalities, such as spasm, dissection, or stenosis, consistent with the idea that the cause of sudden cardiac death was arrhythmic (Fig. 69-2).