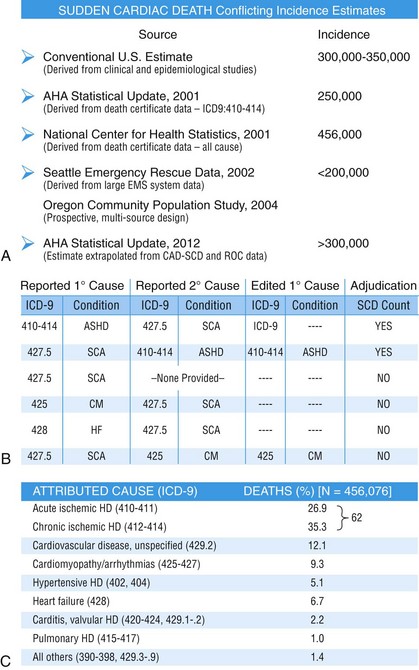
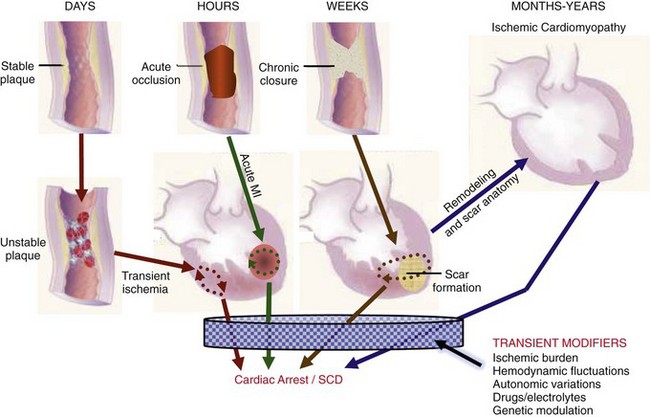
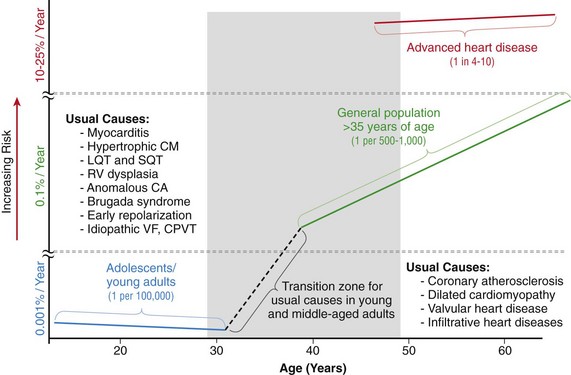
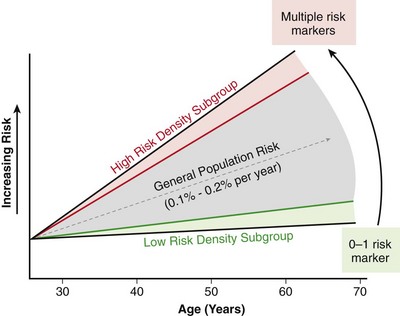
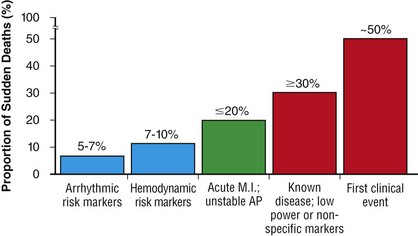
99 Sudden Cardiac Death as a Public Health Burden Structural Substrates and Clinical Expressions Associated With Sudden Cardiac Death Pathologic Substrates in Sudden Cardiac Death Victims Prediction and Strategies for Prevention of Sudden Cardiac Death Prediction of Risk of Sudden Cardiac Death in Coronary Heart Disease Sudden Cardiac Death Risk in Nonischemic Dilated Cardiomyopathy Tachyarrhythmic and Bradyasystolic or Asystolic Cardiac Arrest Mechanisms Inherited Basis of Sudden Cardiac Death Risk in Adults Epidemiologic Paradigms for Sudden Cardiac Death Prediction The generally accepted temporal definition is bracketed by an interval of up to 1 hour between the onset of an abrupt change in clinical status and loss of consciousness.1 This time period defines the onset of SCA used in population studies and clinical analyses, rather than the absolute definition of biologic death, which could be delayed for days to a month or more by life-support interventions after central nervous system injury resulting from the cardiac arrest. For the last 30 years of the twentieth century, the incidence of SCD in the United States has been estimated at 300,000 to 350,000 events per year. These estimates were derived from a broad-based accumulation of clinical and population data available at the time, rather than direct epidemiologic studies providing national statistics that included representation from various regions of the country. During the first decade of the twenty-first century, conflicting statements have been put forward regarding the current incidence of SCD (Figure 99-1, A). These newer estimates were derived from retrospective death certificate analyses and extrapolations from SCD incidences identified in specific communities. For example, an estimate from the American Heart Association’s statistical handbook,2 based on data from the National Center for Health Statistics (NCHS) and the National Heart, Lung, and Blood Institute, suggested an annual incidence of less than 250,000 SCDs. An additional analysis from the Seattle, Washington, emergency medical system database, extrapolated to total U.S. population numbers, calculated 184,000 cardiac arrests nationwide in the year 2000,3 which is a significant decrease compared with 1980 statistics. Another community-based study from Oregon, based on prospective medical examiner data plus other sources of data, also yielded a national extrapolation of less than 200,000.4 In contrast, a death certificate–based study, also from the NCHS, has suggested that more than 50% of all cardiac deaths are sudden and estimated the total annual incidence of SCD to be in the range of 460,000.5 The most recent statistical update from the AHA,6 combining statistics from several sources, returns to the rate of 300,000 to 350,000 SCDs annually. Figure 99-1 Conflicting estimates on the incidence of sudden cardiac death (SCD) in the United States. Recent estimates range from less than 185,000 to greater than 450,000 SCDs annually. A, Estimates over time. Most of the estimates are derived from retrospective analysis of death certificate data, and none is based on a prospective analysis of a population representative of the entire country. Each study used different inclusion criteria. B, The data available in the American Heart Association (AHA) Statistical Handbook are limited to out-of-hospital cardiac arrest with a diagnosis of coronary heart disease, using specific International Classification of Diseases (ICD-9) codes (410 to 414). This source estimated 250,000 SCDs annually in 2001, and in the range of 300,000 to 350,000 in 2012. C, Using a broader range of etiologic definitions for SCD (note the ICD-9 codes used), the National Center for Health Statistics demonstrated that only 62% of all SCDs have a primary diagnosis using the ICD-9 codes on which the AHA estimates are based, and reported an annual frequency of more than 450,000 SCDs. A prospective study using uniform criteria is desirable for clarifying the actual incidence of SCD in the United States. ASHD, Arteriosclerotic heart disease; CM, cardiomyopathy; HD, heart disease; HF, heart failure; SCA, sudden cardiac arrest. (A and B, Modified and updated from Myerburg RJ: Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol 13:709–723, 2002. C, Modified from Zheng ZJ, Croft JB, Giles WH, Mensah GA: Sudden cardiac death in the United States 1989 to 1998. Circulation 104:2158–2163, 2001.) The large discrepancy between the two estimates from NCHS death certificate data stems from differences in definitions of SCD used and population sources. The American Heart Association restricted its application of the NCHS data solely to coronary heart disease–related deaths, based only on International Classification of Diseases–Ninth Revision codes 410 to 414 (see Figure 99-1, B), whereas the cumulative NCHS data included almost all causes of SCD (see Figure 99-1, C). The much broader range of inclusion in the latter study resulted in the much larger estimate, perhaps overestimating the true incidence. The Seattle data include only SCDs involving emergency rescue responses, whereas the Oregon study population is not necessarily representative of the incidence variations within the entire U.S. population. National estimates remain uncertain because of the hazards of relying on death certificate classification and extrapolations from a few large population centers of excellence that might not be representative of smaller metropolitan statistical areas constituting the majority of the U.S. population. This limitation could affect appropriateness of regional response strategies and outcomes, in addition to knowledge of the true population burden. This notion is relevant to the observation that the strongly emphasized reduction in age-adjusted risk of coronary heart disease mortality during the past 40 to 50 years, when translated to total number of events and size of populations at risk for future events, cannot be interpreted to mean that the absolute burden of SCD events has decreased.7 It is likely that general preventive therapies and lifestyle modifications for controlling risk factors for coronary heart disease, plus interventions in acute coronary syndromes, have contributed to a population effect on evolution of disease and the reduction in age-adjusted mortality rates. However, it has also been demonstrated that they have not had a proportional effect on SCD incidence, especially in low-risk subsets with large denominators and high absolute numbers of events. Based on existing data, it appears that the total numerical burden of SCD has not changed significantly over the past 30 years, although underlying disease burden has, and SCD accounts for an increasing proportion of cardiovascular deaths, constituting 50% of cardiac deaths in the United States and Northern Europe.8,9 The limitations in the various sources and reliability of currently available data10 suggest that a prospective epidemiologic study, designed to provide accurate counts of SCDs and its specific mechanisms, including proportional representation from metropolitan statistical areas of different sizes, would be a wise investment to help future health needs planning, community response system designs, and clinical research targets. The etiologic basis for SCD can be partitioned into two general categories: substrate-based causes and expression-based triggers. Substrate-based causation refers to the presence of vascular, myocardial, or molecular substrates that associate with SCD. In parallel, expression risk refers to the identification of mechanisms that contribute to the clinical expression of the risk predisposed by the substrate. In the case of SCD owing to coronary heart disease, these are suggested by the plaque transition and acute syndrome modules in Figure 99-2 (plaque disruption and thrombogenesis), and their subsequent expression as an arrhythmic event (arrhythmogenic module) in the susceptible individual. The arrhythmogenic category of pathophysiology can also be viewed to include modifiers of molecular-based risk that drive individual expression. Figure 99-2 Pathophysiology of cardiac arrest caused by coronary heart disease. Four pathophysiological substrates contribute to arrhythmic risk and sudden cardiac death (SCD) in coronary artery disease. These include transient ischemia, acute coronary syndromes, scar-related pathophysiology, and ischemic cardiomyopathy. The pathophysiology, clinical implications, and long-term risk of each are described in the text. (Modified from Myerburg RJ: Implantable cardioverter-defibrillators after myocardial infarction. New Engl J Med 359:2245–2253, 2008. with permission of the publisher. Copyright © Massachusetts Medical Society, 2008.) Among the general population in the Western hemisphere, coronary heart disease is the most common substrate for SCD. Although estimates vary, it is generally agreed that approximately 75% to 80% of all SCDs in the United States and Western Europe are associated with this underlying substrate.1 The second most frequent category of disorders associated with SCD is cardiomyopathy. Most definitions of this category have included both dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), but the hypertrophic form is more appropriately counted among the rare genetic syndromes associated with SCD risk, for both clinical and epidemiologic reasons. The DCMs collectively account for 10% to 15% of all SCDs. Many studies of SCD risk and interventions fail to distinguish between DCM as an etiology and congestive heart failure (CHF) as a clinical expression. The various infiltrative, inflammatory, and valvular diseases constitute the underlying cause in most of the remaining SCDs. One such disorder is viral myocarditis, which can be associated with SCD whether manifest or silent clinically. Its frequency is uncertain because of the difficulty validating its presence, even histopathologically. It has been considered to be among the more common causes of SCD in adolescents and young adults,11 but its incidence is debated because many of the estimates came from studies that predated the consistent definitions established by the Dallas criteria. More recently, the recognition of persistence of viral DNA fragments in myocytes of SCD victims, even without inflammatory markers, further confounds the issue.12 Only a small proportion of SCDs in middle-aged and older adults are due to the rare genetically determined disorders, such as long QT syndrome, Brugada syndrome, HCM, right ventricular dysplasia, or the developmental congenital disorders, such as aortic stenosis and anomalous origins of coronary arteries. Cumulatively, these rare disorders account for no more than 1% of the total SCD burden, but the proportionate contributions of these disorders are distinctly age-related. Among adolescents and young adults, these disorders (including myocarditis), are dominant causes of SCD, whereas coronary heart disease and the dilated cardiomyopathies dominate beyond the age of 35 years. A transition occurs in the age range of 30 to 45 years, in which the rare disorders comingle with increasing prevalence of coronary heart disease (Figure 99-3). Entities such as Brugada syndrome and right ventricular dysplasia, in addition to long QT syndrome with expression owing to proarrhythmic drug exposures and electrolyte disturbances, are those that are most likely to blend with the progressively increasing prevalence of coronary heart disease and nonischemic cardiomyopathies in this age range. For the general population older than 35 years, the average mortality rate is subgrouped into pockets of higher and lower risk densities, based on numbers and power of risk factors. However, currently available profiling strategies do not have sufficient power to identify small groups with high risk density that would permit meaningful individual risk prediction (Figure 99-4). Figure 99-3 Age-related risk for sudden cardiac death (SCD). A, For the general population 35 years of age and older, the risk of SCD is 0.1 to 0.2 percent per year (1 per 500 to 1,000 population), and the age risk curve is steep (middle curve). For the same population, subgrouped for advanced heart disease, the latter blunts the age-risk relationship (top curve). Among adolescents and young adults, the risk is 1 per 100,000 population, or 0.001% per year, and the age-risk relationship is inverse (bottom curve), with a higher risk at the younger end of the range because of enhanced expression of inherited disorders after puberty. In the age range between 30 and 45 years, a transition occurs in both incidence and the usual causes in the younger adults to those in the middle-aged and those in older adults; evaluation for causes should account for this. (Modified from Myerburg RJ, Castellanos A: Cardiac arrest and sudden cardiac death. In Zipes D, Libby P, Bonow RO, Braunwald E, eds: Heart disease: a textbook of cardiovascular medicine, ed 7. Philadelphia, 2004, Saunders, 2004, pp. 865–908.) Figure 99-4 Stratified risk density curves for the general population older than 35 years of age. The commonly cited average risk in the range of 1 to 2 SCDs per 1000 patient years reflects the cumulative effects of higher and lower risk strata. Identification of the discriminating risk factors would allow for more accurate risk profiling for individual patients within the general population, but current markers of risk do not have sufficient power to yield effect sizes sufficient to significantly improve individual risk prediction. (From Myerburg RJ, Junttila MJ: Sudden cardiac death caused by coronary heart disease. Circulation 125:1043–1052, 2012.) The spectrum of patterns of clinical expression of the SCD syndrome ranges from anticipated events in high-risk patients with identified advanced heart disease to those with unrecognized or new-onset conditions in which SCD is the first clinical manifestation (Figure 99-5). An intermediate category of patients has known heart disease, but is considered to be at low risk based on extent and stability of disease. Among the highest-risk categories are subgroups characterized by low ejection fractions, with or without heart failure, following myocardial infarction or nonischemic cardiomyopathies. Various other myocardial or valvular conditions are also associated with high risk. Among these groups of patients, the probability of a potentially fatal cardiac arrest is high, but the positive and negative predictive accuracies of most methods of profiling risk for individual patients is still somewhat limited.13 At the other end of the risk spectrum, patients with known heart disease but low-risk markers (e.g., ejection fractions greater than 40 percent, no heart failure, no arrhythmias) contribute a large proportion of SCDs. In addition, cardiac arrest is commonly the first clinical manifestation of the underlying disease in subjects without previously identified heart disease. Previously silent disease in such subjects is often advanced. The last two categories account for approximately two thirds of all SCDs. Figure 99-5 Clinical status of sudden cardiac death victims. Effectively predicting cardiac arrest among a high percentage of potential victims is difficult, because approximately 80% of cardiac arrests occur as first clinically manifested events (50%) or in the clinical setting of known disease in the absence of strong risk predictors. Less than 20% of the victims have high-risk markers based on arrhythmic or hemodynamic parameters. Persons at high risk constitute a proportionately smaller segment of the population base. The total exceeds 100% because of overlap between the first event and low risk categories with the acute coronary syndrome group. AP, Angina pectoris; MI, myocardial infarction. (Modified and updated from Myerburg RJ: Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol 12:369–381, 2001.) Much of the information available on the pathologic anatomy in SCD victims reflects the predominance of coronary heart disease as the major underlying etiologic disorder. Both the extent of coronary artery disease and the resulting abnormalities in ventricular myocardium have been well described. It has been recognized for many years that extensive coronary atherosclerosis is the primary anatomic hallmark of coronary heart disease–related SCDs.1 Also recognized in recent years, however, has been a close association of SCD with the presence of plaque fissuring or erosion, platelet aggregation, and acute thrombosis within the matrix of coronary atherosclerosis. This feature of coronary artery anatomy, superimposed on chronic lesions, does not differ among the three primary expressions of acute coronary syndromes—namely, SCD, acute myocardial infarction, and unstable angina pectoris. Various studies have demonstrated the presence of one or more active coronary lesions in up to 90% of SCD victims.14 The active plaque state and its consequences (plaque disruption with hemorrhage into the plaque or intraluminal thrombosis) are more important than the degree of chronic occlusion in the pathophysiology of acute coronary syndromes, including SCD. It has been demonstrated that active plaques are not restricted to the apparent culprit vessel in acute coronary syndromes, including SCD. Thus, a 70% to 80% obstruction without a fresh thrombus, coexisting with lesser lesions in the active state, might not be the true culprit.15 Myocardial pathology reflects the presence and extent of chronic coronary atherosclerosis, in that myocardial scars, owing to healed myocardial infarction, are common.1 Both clinically recognized and unrecognized scars are associated with SCD risk, and the magnitude of risk appears equivalent based on a recent cardiac magnetic resonance imaging study.16 This observation has implications for potential screening techniques for victims in the category of SCDs that are first clinical events. Evidence of acute myocardial infarction is far less common, probably because the pathophysiological mechanism leading to that expression is interrupted by a fatal arrhythmia. However, even among cardiac arrest survivors studied during the prethrombolytic or percutaneous coronary intervention era, evolution to acute transmural myocardial infarction was less common. An association between the number of involved coronary arteries and the extent of ventricular hypertrophy in SCD victims also has been recognized. A correlation between extensive coronary artery disease and left ventricular hypertrophy might be a consequence of longstanding hypertension as a risk factor in coronary heart disease patients or of post–myocardial infarction remodeling. Regardless of the mechanism, myocardial scars and left ventricular hypertrophy are considered factors in arrhythmogenesis during transient ischemia.1,7
Sudden Cardiac Death in Adults
Sudden Cardiac Death as a Public Health Burden
Structural Substrates and Clinical Expressions Associated with Sudden Cardiac Death
Pathologic Substrates in Sudden Cardiac Death Victims
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
