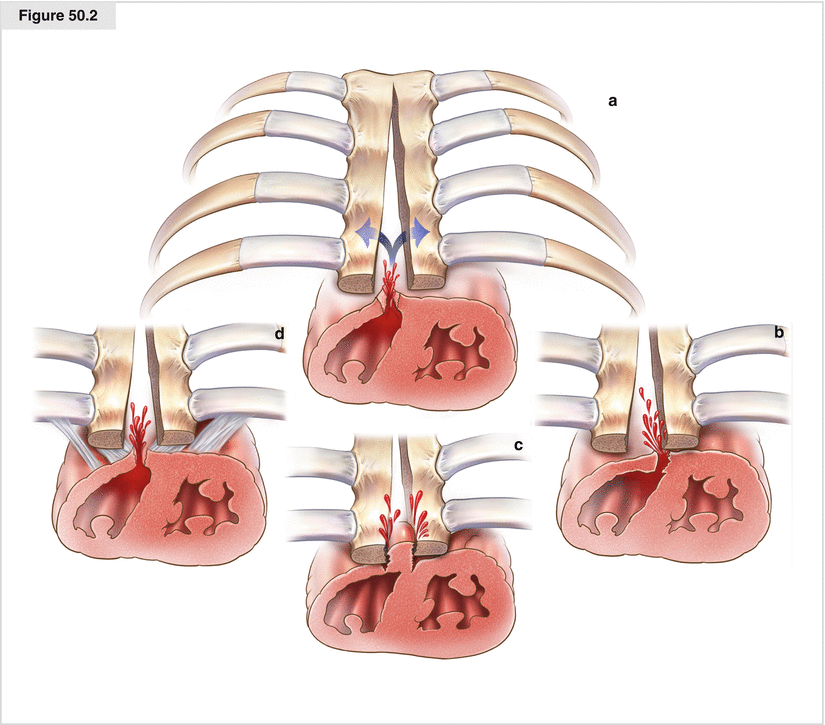
Figures 50.1 and 50.2
(a–d) The most important step is radical surgical débridement with complete removal of all infected and necrotic material. This step also includes removal of all wires or other foreign material, as continued infection positively maintains necrosis, destroying tissue needed for myoplastic coverage. If this occurs, there is no chance the infection will heal. This step also is necessary to dislodge all infected and necrotic osseous material. Partial or total sternal resection must be performed if necessary. It is important to preserve and fix vital sternal remains because they sustain thoracic stability and impede respiratory insufficiency. Following this procedure, the whole wound must be bowdlerized mechanically and by jet lavage. Swabs are taken routinely; as a complementary procedure, systemic antibiotic therapy is initiated based on antibiogram results and infection progression. If the wound conditions are perfect, closure may follow directly. Otherwise, VAC therapy is recommended. The sponge is trimmed as small as possible to prevent shrinkage of soft tissue, which makes reconstruction more difficult. Surgical débridement and VAC should be performed until the wound is decontaminated and ready for reconstruction; a low bacterial count is acceptable (Fig. 50.1, path I). Special conditions exist in patients who had prior cardiac surgery, especially more recent surgery. Patients readmitted within 6 weeks after cardiac surgery are most endangered and receive therapy according to path II. Rupture of the right ventricle may occur as the result of a different etiology, such as:
1. Continued spread of infection
2. Sharp sternal edges
3. Increased intrathoracic pressure or loss of vacuum, either accidental or because of system disconnection
4. Adhesions between the heart and chest wall
Despite the stabilizing subatmospheric pressure from VAC therapy, surgical intervention may result in right ventricle rupture due to the aforementioned causes (Fig. 50.2). Sternal refixation or placement of a layer between the heart and sternum is mandatory to avoid damage to the heart and is imperative after each surgical débridement. We prefer refixation, as it ensures that mediastinal organs are not damaged by the sternum or its remains. With this approach, wires and cords must be removed and applied again after each débridement; however, complete rewiring is unnecessary as three to four cerclages or polydioxanone cords are sufficient. This applies to the ongoing VAC therapy as well as to the reconstructive procedures. As a result of the punctual fixation, the mediastinum may be drained easily while safety is maximized. Additionally, unwanted pressure losses due to accidental dressing removal or system disconnection may be fixed easily without subjecting the patient to the risk of ventricular rupture. This procedure is postponed until consolidation and subsequent plastic reconstruction. After consolidation, direct closure or, in most cases, myoplastic reconstruction may be undertaken. If more than 6–8 weeks have passed between sternotomy and infection treatment, radical surgical débridement with wire removal is performed. Thus, rewiring is not necessary in most cases. Ample connective tissue regeneration and a fixed ledge above the heart are required. Stabilization is achieved by applying a connective tissue plate and VAC. With the patient’s consent, pseudoarthrosis may be appropriate. After consolidation, myoplastic reconstruction is performed (Fig. 50.1, path III)
Reconstruction
Reconstruction may be achieved using autologous or synthetic tissue. Muscle flap options for reconstruction of anterior chest wall defects due to sternal infections are:
1.
Greater pectoral muscle flap, unilateral or bilateral, with or without deinsertion of the tendon insertion (advancement, turnover, transposition flap)
2.
Greater pectoral–rectus abdominis continuous flap
3.
Greater omentum flap
4.
Latissimus dorsi flap
5.
Rectus abdominis flap (pedicled, free)
6.
Tensor muscle of fascia lata flap (free)
7.
Anterolateral thigh flap (free)
Because of the plentitude of nearby tissue, most defects due to sternal infections can be reconstructed with local tissue, in most cases with a greater pectoral muscle flap. This approach undoubtedly is more beneficial than using synthetic tissue. The following figures depict surgical procedures according to their frequency of application.
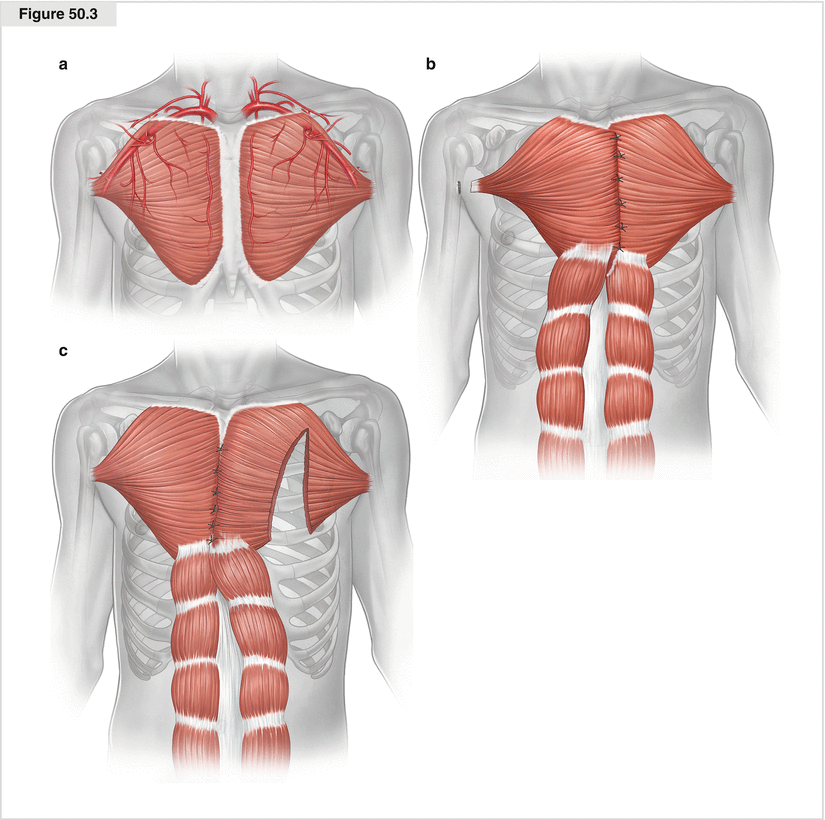

Figure 50.3




(a–c) Greater pectoral muscle flap. The favored muscle for covering the sternum via tissue transfer is the greater pectoral. Its main blood supply is from the thoracoacromial trunk, but it also is supplied by segmental perforators from the lateral thoracic and internal mammary arteries. The greater pectoral muscle originates at the aponeurosis of the external oblique, anterior sternum, second to sixth costal cartilages, and medial half of the clavicle. It inserts at the lateral lip of the bicipital groove by a flat trilaminar tendon. Its functions are flexion, adduction, and internal rotation of the arm, and it serves as the external respiratory muscle. Greater pectoral muscle flaps may be myocutaneous, osteomyocutaneous, pedicled, or free. Different techniques are possible for mobilizing the flap. The patient is placed in the supine position with the arms prepared for eventual deinsertion. The flap is harvested unilaterally or bilaterally. The muscle is dissected from the sternal origin in continuity with the subcutaneous fatty tissue if the surgeon does not want to dissect the insertion. The muscle and fatty tissue are dissected separately if the surgeon is going to dissect the insertion at the humerus. Cautery is used to raise the full length of the muscle from its sternal origin, then the pedicle is dissected from the clavicular part as far as necessary. If the sternal origin is dissected, further preparation may be done mostly bluntly up to the armpit. The perforators must be cauterized carefully. This way of harvesting the flap on both sides should be sufficient for an advancement flap. If more length is needed, unilateral or bilateral dissection of the insertion is required. An axillary incision adjacent to the insertion is helpful. Additional length is provided by dissecting the flap from its costal origins. In this way, the pedicle can be completely isolated on the thoracoacromial trunk. Another method of facilitating the stressless midline closure of the muscles is splitting the pedicle in the middle part longitudinally, taking the blood supply into account. For a unilateral harvest of greater pectoral muscle flap, the nondominant arm is preferred. To prepare a turnover flap, the pedicle is dissected from the insertion at the humerus, the costal parts, and the blood supply of the thoracoacromial branch and also is raised from the clavicular part. The blood supply is guaranteed by the perforators of the internal mammary artery. In this way, the pedicle can be turned into the sternal space. This procedure is possible only with an intact ipsilateral mammary artery. Because of its location, distinct blood supply, and excellent rotational ability, the muscle offers the possibility of covering at least two thirds of the sternum. In the case of extensive defects, particularly those involving the lower third of the sternum, the greater pectoral muscle can be raised in continuity with the rectus abdominis to cover the whole defect. The ipsilateral internal mammary artery should be intact if this approach is taken. Donor site morbidity is low, and there is no functional defect. As a reconstructive method after surgical débridement, greater pectoral musculoplasty has multiple advantages. There is no need for an additional entry method; surgical complexity is low, with little donor site morbidity; and the cosmetic results are good. However, turnover flaps might be bulky
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


