55 Stem Cell Therapy for Ischemic Heart Disease
 Introduction
Introduction
The many efforts to maximize therapy for the treatment of patients with acute myocardial infarction (AMI) have yielded significant benefits. Beginning first with thrombolytic therapy for AMI and more recently with the availability of primary percutaneous coronary intervention (PCI) for ST-segment elevation MI, the mortality rates of this devastating ischemic event have decreased from almost 15% in clinical trials in the late 1980s 1 to under 5% in recent primary PCI trials.2 Prior to these advances, ischemic heart disease was the leading cause of chronic heart failure. While further improvements in reperfusion are needed, the current advances have led to the growing epidemic of heart disease, with many patients surviving what in the past might have been fatal events. With these advances, the prevalence of chronic heart failure (CHF) has increased dramatically over the preceding decade, with now more than 10% of the U.S. population over 65 years of age carrying this diagnosis. While the mechanisms are still under investigation,3,4 the development of chronic heart failure following MI is more than just the loss of contractile tissue but also partly determined by the ventricular remodeling that occurs in response to myocardial necrosis.5 The inflammatory response to myocardial necrosis leads to infarct expansion, dilation of the left ventricular (LV) cavity, and replacement of cardiomyocytes with fibrous tissue.3,5 Currently available therapies to alter the remodeling process and the progression to chronic heart failure remain limited, and death rates from this condition continue to rise. Based on current trends the problem is predicted to increase to greater than 6 million people by the year 2030. The increasing burden of chronic heart failure has been addressed with pharmacological therapy, which in some can delay and improve the morbidity and mortality of heart disease; electrical therapy, including cardiac resynchronization therapy; LV remodeling surgery; and, with the approval of the first LV assist device for destination therapy, mechanical therapy. Given the shortcomings of each of these strategies and the fact that none of them address the underlying problem—loss of cardiac myocytes due to ischemic death—many have turned to molecular therapy, and more specifically stem cell-based therapy, as the hope for the future.
 Approaches to Cell Therapy
Approaches to Cell Therapy
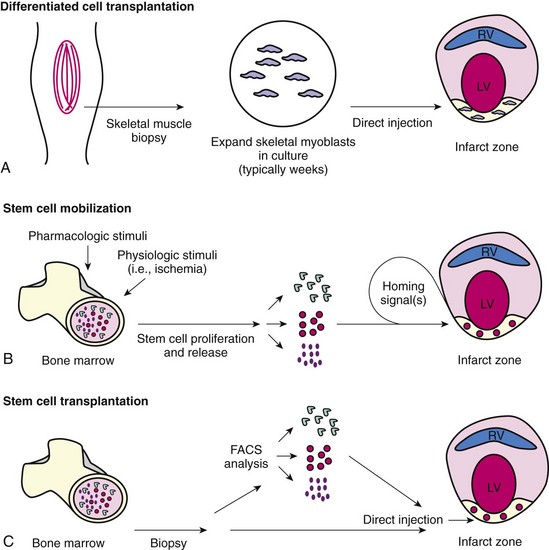
The field of cell transplantation for the treatment of left ventricular dysfunction following ischemic injury continues to make significant progress, with dozens of studies now completed and metanalyses suggesting overall benefit to those patients who are treated compared with controls.6,7 Theoretically, optimal cell therapy will use cell types that possess the capacity to incorporate themselves into the recipient myocardium and that will be able to survive, mature, and couple electromechanically with each other and native cardiac myocytes. The field began with differentiated cell transplantation (Fig. 55-1A), the goal of which was to functionally achieve myoplasty through the transplantation of autologous skeletal myoblasts that would engraft into injured myocardium in patients with chronic ischemic heart failure.8 Another strategy that has been implemented clinically is stem cell mobilization (Fig. 55-1B), which involves the pharmacological mobilization of a patient’s own bone marrow cells into the bloodstream, the idea being that these cells would engraft into areas of myocardial damage and then direct the engrafted stem cells to differentiate into cardiac myocytes and vascular structures that would fully integrate themselves with the native myocardium. By far the most comprehensively studied strategy to date that has shown some benefits is stem cell transplantation (Fig. 55-1C), which involves the removal of stem cells from the patient’s bone marrow (autologous cells) or a well-screened donor (allogeneic cells) and then injecting the whole bone marrow or a selected population of bone marrow-derived stem cells into the infarct zone. Questions abound as to how these strategies will move forward and lead to clinical benefit in preventing (treatment in patients in the peri-infarct period) or treating (treatment in patients with chronic heart failure) cardiac dysfunction. Below we summarize the current state of knowledge and studies that are ongoing while also considering what their findings may mean and where the field may be moving.
Differentiated Cell Transplantation
The goal of differentiated cell transplantation is to replace scarred myocardium with living viable cells, ultimately leading to an overall improvement of myocardial function. A number of cell types, including smooth muscle cells and skeletal myoblasts, have been studied as potential candidates for differentiated cell transplantation. The value of these cell types is due to their accessibility and ability to be expanded in vitro prior to transplantation. Arguably the first strategy of cell therapy for chronic heart failure was cardiac myoplasty (cardiomyoplasty). During this procedure, the heart was wrapped in skeletal muscle that was then paced to increase the contractility of the weakened heart.9–11 It was recognized that there was engraftment of skeletal muscle in the epicardial layers of the heart,12,13 which we now recognize was the ingrowth and engraftment of skeletal myoblasts.14 Following these observations, the early preclinical and clinical studies with skeletal myoblasts set the stage for the potential benefits associated with the delivery and engraftment of exogenous cells into the heart to prevent and treat cardiac dysfunction. We learned that exogenous cells could engraft into the heart and that the engraftment of skeletal myoblasts could lead to clinical benefit, including inducing reverse LV remodeling.15–17 We further learned the importance of the mechanical coupling of exogenous cells to the cellular milieu of the heart.18 Skeletal myoblasts (SKMBs) do not integrate into the electrical syncytium of the myocardium and have been shown in animal studies to induce slow conduction,19 increased risk of reentrant rhythm,20 and increased premature ventricular contractions and ventricular tachycardia in patients.8 It takes at least 2 to 3 weeks between skeletal muscle biopsy and the availability of a sufficient number of SKMBs for transplantation in humans. Thus, while SKMBs have some desirable properties for cell therapy, the timing of delivering SKMB treatment compared with the stem cell approaches is problematic. This timing issue, the lack of vascular growth induced by SKMBs alone,21 and the proarrhythmogenic effect of SKMBs makes debatable the issue of whether SKMB transplantation alone will offer significant clinical benefit.
Myocardial Regeneration
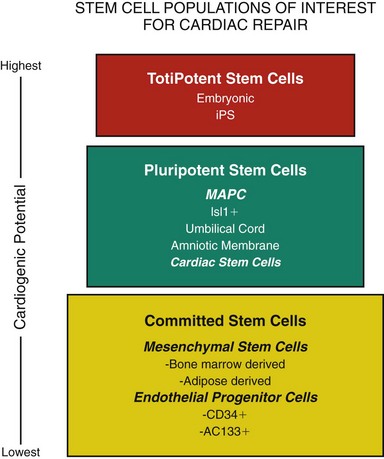
Since the turn of the century, a significant body of literature has rewritten the once strongly held belief that the heart cannot repair itself. We have learned that following MI in humans there is a transient mobilization of stem cells and expression of stem cell homing factors that recruit these cells to the heart.22,23 Human female hearts transplanted into males were found to have cardiac myocytes and vascular structures that stained positive for the Y chromosome, suggesting that these cells originated from the stem cells of the transplant recipient.24 Unfortunately these studies demonstrate that stem cell engraftment and differentiation into cardiac myocytes is an infrequent event (0.02% cardiac myocytes, 3.3% endothelial cells). However, these studies do suggest that the normal physiological response to myocardial injury is mobilization of stem cells, “homing” of these cells to the damaged myocardium, and differentiation of at least some of them into cardiac myocytes. Furthermore, if this natural repair mechanism can be potentiated, clinically meaningful myocardial regeneration may be achievable. The excitement surrounding the use of stem cells is based on the unique biological properties of these cells and their capacity to self-renew and regenerate tissue and organ systems. Figure 55-2 groups different stem cell populations of interest in myocardial regeneration based on their cardiogenic potential. While it was once believed that all stem cell populations listed in Figure 55-2 could differentiate into cardiac myocytes,25,26 it is now clear that while many of these cell populations may lead to improved cardiac function,25,27 following transplantation, only a limited number of them become cardiac myocytes.28,29 Importantly, recent studies using mesenchymal stem cells derived from the amniotic membrane demonstrate that the delivery of cardiogenic stem cells results in significant improvements in cardiac function despite the lack of significant regeneration of structural myocardium.30,31
Bone Marrow-Derived Mononuclear Cells
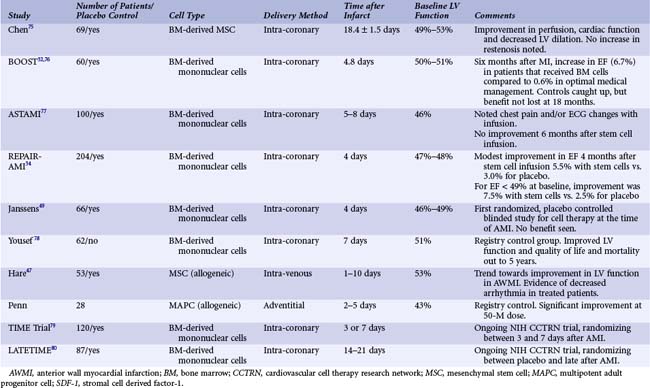
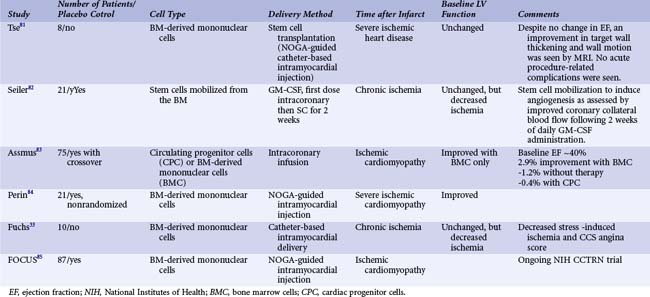
The transplantation of bone marrow-derived mononuclear cell preparations to the infarct-related vessel has progressed to the point where data from randomized controlled trials are now becoming available for patients with AMI (Table 55-1) and CHF (Table 55-2). The first randomized stem cell trial for patients with AMI was the BOOST trial.32 These trials can be summarized as follows:
As seen in Table 55-1, while this strategy has not always resulted in improved cardiac function, most trials have demonstrated a significant increase in cardiac function ranging from 2% to 6%. Importantly, in some trials the early benefits seen with cell therapy were lost with subsequent follow-up, suggesting that the infusion of bone marrow mononuclear cells accelerates healing of the heart but may not further improve it.33 In contrast, serial follow-up from the largest bone marrow infusion trial to date, the REPAIR-AMI study,34 found continued improvement in cardiac function as well as continued trends in benefit in clinical parameters including hospitalization for heart failure, reinfarction, and survival. At the 4-month time point, the treated population had an increase in ejection fraction of 6% compared with baseline versus the control population, which exhibited only a 2.5% increase. In more recent follow-up studies, these benefits and evidence of decreased clinical events have been maintained out to 2 and 5 years after treatment.35 There are several ongoing clinical studies using bone marrow-derived mononuclear cells in the peri-MI period that will address important questions. The Cardiovascular Cell Therapy Research Network, an NIH-funded network of clinical sites, is running the TIME and LateTIME studies. These are addressing the important question of the optimal timing for cell therapy. The TIME study is randomizing patients between cells and placebo and treatment 3 or 7 days after primary PCI. The LateTIME study is randomizing between cells and placebo at 2 to 3 weeks after primary PCI. These studies will further suggest optimal timing of bone marrow mononuclear cell treatment as well as beginning to define when is too late for treatment.
 Novel Autologous Stem Cell Indications and Types
Novel Autologous Stem Cell Indications and Types
Two recent studies have demonstrated the potential of two specific autologous cell populations that significantly advance the field of cardiovascular cell therapy. The first is the ACT-34 study. This study, a randomized double-blind placebo-controlled trial, focused on patients with chronic myocardial ischemia. In this population, a placebo-controlled blinded study was performed in which patients received granulocyte-colony stimulating factor (G-CSF) mobilization followed by apheresis and isolation of CD34+ cells. These cells were injected via an endoventricular approach using the NOGA catheter. The recent release of the 1-year follow-up data for this patient population revealed a decrease of 5.6 angina events per week and a doubling of exercise treadmill time versus baseline. These data verify in the clinical setting the preclinical findings that stem cell-based cardiac repair results in a significant and sustained increase in vascular density, leading to improved perfusion of the myocardium. A second trial focused on the effects of cardiac stem cells in patients with ischemic cardiomyopathy. Based on the relative dosing of stem cells in preclinical trials, it would appear that cardiac stem cells may be the most potent of all the adult stem cells.36 If true, then cardiac stem cells may be the best cells to use for stem cell transplantation (Fig. 55-1) as an adjunct to open heart surgery in the setting of coronary grafting, valve surgery, or left ventricular assist device implantation. Just as adipose-derived stem cells can be rapidly harvested from adipose tissue,37,38 it may become possible to rapidly isolate cardiac stem cells from the left atrial appendage in the operating room or from endomyocardial biopsies in sufficient number to allow for meaningful myocardial support. In the SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) trial (Clinicaltrials.org; NCT00474461), cardiac stem cells were isolated from atrial tissue obtained at the time of coronary artery bypass surgery. These cardiac stem cells were cultured and, after sufficient propagation, were injected into injured myocardium through a percutaneous approach Thus far, data from this trial have demonstrated varied results but, on average, a ~9% increase in cardiac function. While preliminary, this trial is a good example of the novel approaches being undertaken to investigate and optimize adult stem cell therapy.
Mesenchymal Stem Cells and Multipotent Adult Progenitor Cells
Perhaps the greatest recent advance in cardiovascular cell therapy has been the successful implementation of allogeneic stem cell sources in the treatment of patients with AMI. Stromal progenitor cells comprise less than 0.05% of the adult bone marrow. Mesenchymal stem cells (MSCs) and multipotent adult progenitor cells (MAPCs) from subpopulations of bone marrow stromal progenitor cells maintain the ability to differentiate along multiple lineages.26,39 MSCs can be immunoselected via cell-surface CD45 negativity and cultured through as many as 40 population doublings before attaining senescence. In general the benefits observed with MSCs following cell transplantation at the time of MI may have little to do with the cell itself but more with how factors secreted by the MSCs alter the tissue microenvironment. This so-called paracrine effect of MSCs has been demonstrated,40 showing that injection of the supernatant of MSC cell cultures at the time of MI results in benefits similar to those that have been associated with MSC cell transplantation. More recently we have demonstrated that stromal cell-derived factor 1 (SDF-1) released by MSCs results in the recruitment of cardiac stem cells to the infarct border zone while also inhibiting cardiac myocyte death through the binding of SDF-1 to CXCR4-expressing cardiac myocytes in the infarct border zone. Recent preclinical studies have demonstrated that modulation of wnt/b-catenin signaling through TGF-β pretreatment or more precisely by modulating disabled-2 induce MSCs to increase expression of proteins in vitro that are normally expressed by cardiac myocytes, leading to an improved functional response.41 MAPCs are stromal cells derived from the bone marrow; they are able to differentiate into endothelial, epithelial, and mesenchymal cell types.42 They can be expanded in culture for more than 80 population doublings and still maintain their pluripotency by differentiating into most somatic cell types.42 Whether these multipotent stem cells exist in vivo or are the result of serial cell passages in culture remains to be determined. However, the demonstration that multipotent cells can be significantly expanded as well as the benefit they have shown in early animal studies43 bode well for the development of future therapies. Both MSCs and MAPCs have been shown to lead to decreased cardiac myocyte cell death, decreased infarct size, improved cardiac remodeling, and improved function in rodent44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree