CHAPTER 8
Stage C: Improving Outcomes in Symptomatic Heart Failure
“There are in fact, two things: science and opinion; the former begets knowledge, the latter ignorance.”
—Hippocrates
Evidence-Based Therapies for Patients with HF-rEF
The basic algorithm for treating patients with HF-rEF is based on both the severity of symptoms and the degree of LV dysfunction (Figure 8.1). Pharmacologic therapies target different neurohormonal systems.
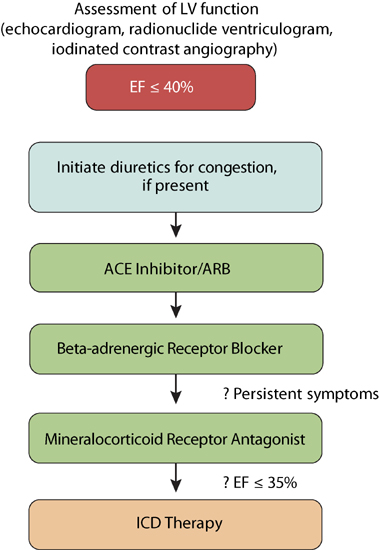
FIGURE 8.1 Steps in treating patients with HF-rEF.
EVIDENCE-BASED THERAPIES REDUCE RISK
Optimal implementation of six evidence-based therapies improves survival in heart failure patients (Table 8.1, Appendix B). A proportion of patients, however, are currently undertreated (Table 8.2).1 There may be several reasons for inconsistent application of evidence based guidelines, but hopefully, an explicit understanding of beneficial effects when consistently applying these recommended strategies, may lead to increased use in practice. Maximizing patient therapy could contribute to a decrease in heart failure symptoms and associated hospitalizations, and if mortality risk reductions of each modality are additive, then complete implementation of all 6 therapies has been estimated to save up to 67,996 deaths per year.1 Commonly used doses of heart failure medications are listed at the end of this chapter (Table 8.4, p. 197).
TABLE 8.1 Mortality risk reduction for each component of heart failure therapy.1
THERAPY | MORTALITY RELATIVE-RISK REDUCTION | CLINICAL TRIALS |
ACEI/ARB | 17% | The SOLVD Investigators (Studies of Left Ventricular Dysfunction) |
BETA-BLOCKER | 34% | COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival Trial) MERIT-HF Investigators (Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure) |
ALDOSTERONE ANTAGONIST | 30% | RALES (Randomized Aldactone Evaluation Study Investigators) EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) |
HYDRALAZINE/NITRATE | 43% | A-HeFT (African-American Heart Failure Trial) |
ICD | 23% | SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) |
CRT | 36% | COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) CARE-HF Study Investigators (Cardiac Resynchronization-Heart Failure) |
TABLE 8.2 Eligible heart failure patients not treated for guideline-recommended therapy.1 (Not all patients with heart failure are eligible for every type of treatment.)
THERAPY | TOTAL PATIENT POPULATION WITH HF ELIGIBLE FOR TREATMENT | ELIGIBLE HF POPULATION NOT TREATED | POTENTIAL ADDITIONAL LIVES SAVED PER YEAR IF TREATED |
ACEI/ARB | 2,459,644 | 20.4% | 6,516 |
BETA-BLOCKER | 2,512,560 | 14.4% | 12,922 |
ALDOSTERONE ANTAGONIST | 603,014 | 63.9% | 21,407 |
HYDRALAZINE/NITRATE | 150,754 | 92.7% | 6,655 |
ICD | 1,725,732 | 49.4% | 12,179 |
CRT | 326,151 | 61.2% | 8,317 |
Volume Management with Diuretics
Most patients with a history of pulmonary congestion require a loop diuretic such as furosemide. Some patients with mild volume overload can maintain euvolemia with sodium restriction alone or in conjunction with a thiazide diuretic.
STRATEGIES FOR IMPROVING THE RESPONSE TO DIURESIS
If volume overload persists, changing a patient from furosemide to bumetanide or torsemide may enhance diuretic response (see below). In addition, intravenous administration avoids problems with bioavailability and improves efficacy. In other cases, as a “booster” for loop diuretics, oral metolazone 2.5–5 mg or hydrochlorothiazide 6.25–25 mg can be added every one to three days. An afternoon second dose of loop diuretic may be helpful.
Generic Name • Furosemide • Bumetanide • Torsemide • Ethacrynic acid | Brand Name • Lasix® • Bumex® • Demadex® • Edecrin® | Equivalent IV Doses • 40 mg • 1 mg • 20 mg • 50 mg |
LOOP DIURETICS: TORSEMIDE COMPARED TO FUROSEMIDE
The relative effectiveness of oral loop diuretics depends in part on differences in bioavailability and duration of action. Oral torsemide compared with furosemide has a greater bioavailability (80%–100% vs. 10%–90%), shorter onset of action (Tmax = 1.1 h vs. 2.4 h), and a longer duration of action (18–24 h vs. 4–6 h). Moreover, food intake can reduce furosemide’s bioavailability, but does not affect torsemide.2 Doses for effective diuresis vary among patients. One strategy is to titrate by doubling the dose of oral furosemide (up to 160 mg/day) or bumetanide (up to 4 mg/day), with a low threshold to change to torsemide (up to 200 mg/day) when a patient has persistent congestion. Fewer differences are seen among loop diuretics when administered intravenously.
Angiotensin II Inhibition
At the systemic level, angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) act as vasodilators by reducing the vasoconstricting effects of angiotensin II. Short-term, the arterial and venous vasodilation effects may contribute to improvements in symptoms of low cardiac output and congestion, respectively, unless offset by the effects of hypotension. Angiotensin II can also be generated from angiotensinogen locally in both the cardiac interstitium and intracellular compartments.3 Long-term benefits of ACE inhibitors on heart failure outcomes may relate to reducing the local effects angiotensin II on organ function and structure as well as renal mechanisms.
CONTRAINDICATIONS AND PRECAUTIONS FOR ACE INHIBITORS
Patients with heart failure due to left ventricular systolic dysfunction should be given a trial of ACE inhibitors, unless they have experienced life-threatening adverse reactions during previous medication exposure or if they are pregnant or plan to become pregnant. Other conditions that may be relative contraindications include low systemic blood pressure and renal dysfunction. Also consider alternatives to ACE inhibitors if a patient has a very low systemic blood pressure (systolic blood pressure < 80 mm Hg), markedly increased serum levels of creatinine (> 3 mg/dL), bilateral renal artery stenosis, or elevated levels of serum potassium (> 5.0 mEq/L). ACE inhibitors can be reconsidered when these parameters have improved. In the case of persistent renal insufficiency, half the usual dose can be considered. When treating a patient with ACE inhibitors, an increase in creatinine up to 0.5 mg/dL can be acceptable if blood pressure is maintained.
SIDE EFFECTS
Although all ACE inhibitors lead to decreased production of angiotensin II, side effects may differ by specific agent. Any drug from this class can cause hypotension, cough, renal insufficiency, angioedema, and dysgeusia (change of taste). Agents with sulfhydryl groups, such as captopril, may also cause neutropenia, rash, and proteinuria.4 ARBs have similar efficacy without the side effect of cough. Although angioedema can occur with ARBs, the incidence is much less than with ACE inhibitors.5
CLINICAL TRIAL DATA FOR ACE INHIBITORS
Studies of LV Dysfunction (SOLVD) Trial
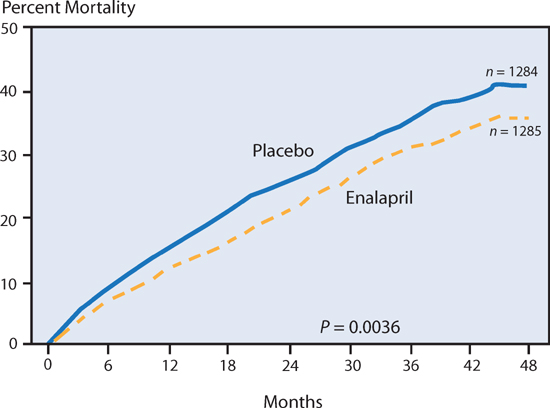
The SOLVD Trial found that HF patients have improved survival beginning almost at the time of initiation of therapy with the ACE inhibitor enalapril (Figure 8.2). In this study, enalapril (target dose 10 mg twice daily) was compared to placebo in patients with heart failure symptoms and an EF ≤ 35%. The survival difference between patients receiving enalapril versus placebo increased over time.6
FIGURE 8.2 The SOLVD trial and heart failure survival with enalapril therapy. From the SOLVD Investigators, effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure, further details in text.6 Source: Reprinted with permission from N Engl J Med. 1991;325(5):293-302. Copyright © Massachusetts Medical Society. All rights reserved.
Achieving Target Doses with ACE Inhibitors
Patient benefits improve when target doses of ACE inhibitor therapy are reached. The Assessment of Treatment with Lisinopril and Survival (ATLAS) trial found a dose-related response to ACE inhibition, achieving a better efficacy and cost-effectiveness with lisinopril goal of 32.5–35.0 mg per day compared to lower doses of 2.5–5.0 mg per day.7 In most patients, a target of 20 mg per day of lisinopril or equivalent is desirable. If hypotension occurs during titration of ACE inhibitor dose, decreasing it may be necessary to decrease the diuretic dose.
Captopril Blunts Post-MI Remodeling
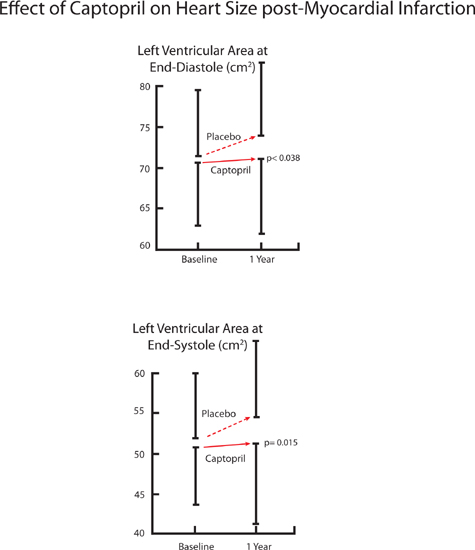
In the Survival and Ventricular Enlargement (SAVE) trial,8 patients who received the ACE inhibitor captopril had smaller increases in both left ventricular end-diastolic and end-systolic dimension at 1 year compared to placebo (Figure 8.3). During a mean follow-up of 3 years, captopril decreased adverse cardiovascular events (cardiovascular death, heart failure requiring hospitalization, or recurrent myocardial infarction). These findings demonstrate the mechanism of ACE inhibition: preventing adverse remodeling changes in left ventricular size and structure improves outcomes in heart failure post-MI.
FIGURE 8.3 The SAVE trial: ACE inhibitors blunt increases in heart size after myocardial infarction. Left ventricular area measured by 2-dimensional echocardiography (Captopril, n = 203; Placebo, n = 217).8 Source: Adapted with permission from St. John Sutton et al., Circulation. 1994;89(1):68-75.
ACE INHIBITORS (ACEI) VERSUS ANGIOTENSIN II RECEPTOR BLOCKERS (ARBs)
Angiotensin II receptor blockers (ARBs) may be an alternative to ACE inhibitor therapy. Although use of either ACE inhibitors or ARBs will decrease the deleterious effects of angiotensin II, only ACE inhibitors will increase the level of the vasodilator bradykinin by blocking its degradation. Although bradykinin may have beneficial effects, including the release of nitric oxide and prostacyclin, it may also contribute to side effects such as angioedema and cough. Because ARBs do not affect bradykinin levels, these side effects are uncommon. Presently available ARBs block the angiotensin II type-1 receptor (responsible for the direct cardiovascular effects of angiotensin II) and not the type-2 receptor (possibly a desirable mediator for prevention of apoptosis).5
Thus, ARBs have related, but not equivalent, effects to ACE inhibitors. Nevertheless, clinical outcomes in heart failure with either agent have been similar. When the ARB candesartan was assessed in patients who were previously intolerant to ACE inhibitors, compared to placebo, candesartan significantly reduced cardiovascular death and hospital admission for heart failure by 30%, similar to the beneficial effects expected with ACE inhibitors (Figure 8.4).9
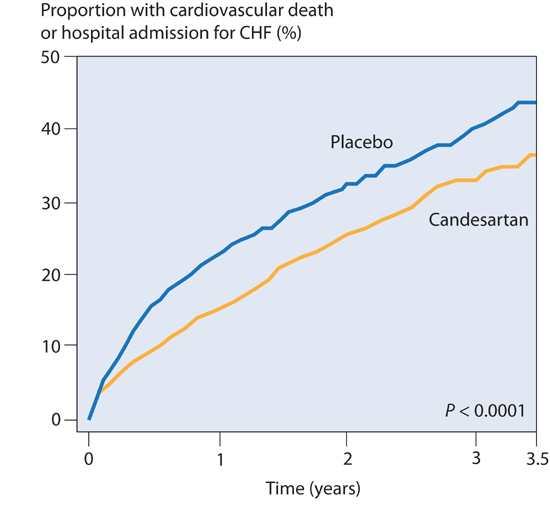
FIGURE 8.4 CHARM trial: ARB therapy for HF-rEF. The ARB candesartan, used in patients not on an ACE inhibitor, reduced cardiovascular death or hospital admission (n = 2028; hazard ratio (HR), 0.77).9 Source: Adapted with permission from Granger et al., Lancet. 2003;362(9386):772-776.
INDICATIONS FOR ACE INHIBITORS AND ARBs
The indications for ACE inhibitors and ARBs have increased over time. Initially, ACE inhibitors were identified as treatment of symptomatic left ventricular systolic dysfunction. Subsequently, ACE inhibitors and ARBs were found to improve outcomes in three additional related categories of patients:
1. asymptomatic patients with ejection fractions ≤ 35% due to either ischemic or nonischemic cardiomyopathy6,9
2. patients 3–16 days following ST-elevation myocardial infarction (STEMI) with ejection fractions < 40%10,11
3. patients presenting within the first 24 hours after STEMI12,13
NO BENEFIT TO COMBINATION THERAPY WITH ACE INHIBITOR AND ARB
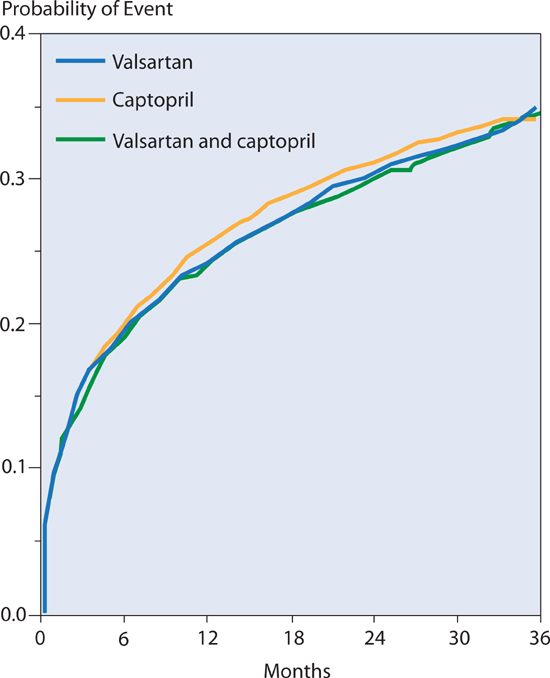
Several large, multicenter trials (Val-HeFT: Valsartan Heart Failure Trial; CHARM: Candesartan in Heart Failure Assessment of Reduction in Morbidity and Mortality; and VALIANT: Valsartan in Acute Myocardial Infarction) have failed to find benefit of adding an ARB to patients on ACE inhibitor therapy. In the VALIANT trial,11 valsartan, captopril, or both led to equivalent rates of death and other adverse cardiovascular outcomes in patients with myocardial infarction complicated by heart failure or left ventricular ejection fraction ≤ 35% (Figure 8.5). Thus, most patients with HF-rEF will benefit from either an ACE inhibitor or ARB, but not both.
However, addition of a mineralocorticoid receptor antagonist to either an ACE inhibitor or an ARB should be considered in patients with HF-rEF (see p. 181). Triple therapy with an ACE inhibitor, ARB, and mineralocorticoid receptor antagonist should be avoided because of no added benefit and increased risk of hyperkalemia and renal dysfunction.14
FIGURE 8.5 VALIANT trial comparing either ACEI, or ARB, or both therapies. Cardiovascular morbidity and mortality comparison between either valsartan or captopril alone, or with combined therapy in patients with MI complicated by heart failure or left ventricular systolic dysfunction. No benefit of combined therapy was seen compared to single drug use.11 Source: Adapted with permission from Pfeffer et al., N Engl J Med. 2003;349(20):1893-1906.
DIRECT INHIBITION OF RENIN
The direct renin inhibitor aliskiren (inhibits the enzyme renin, and cleavage of angiotensin I from angiotensinogen) has been approved for treatment of systemic hypertension. The possible use of aliskiren as an alternative or supplemental therapy to an ACE inhibitor or ARB for heart failure is uncertain at this time pending the results of randomized trials.15
Beta Adrenoreceptor Blockade
Use of beta-blockers for the treatment of either ischemic or nonischemic cardiomyopathy has a history of initial skepticism followed by gradual acceptance for treatment of disease progression in patients with systolic dysfunction, regardless of symptom status.14
Beta-blockers can be classified as first, second, or third generation based on specific pharmacologic properties. First generation beta-blockers include propranolol with nonspecific blockade properties of both β1- and β2-catecholamine receptors. Second generation beta-blockers include metoprolol, bisoprolol, or atenolol, specific for the β1-receptor subtype. Third generation beta-blockers include carvedilol, nebivolol, and labetalol and have additional mechanisms of cardiovascular activity such as vasodilatation. The beta-blockers carvedilol, extended-release metoprolol, and bisoprolol improve survival in patients with symptomatic systolic dysfunction (HF-rEF). Of these, the data for survival benefit are strongest for carvedilol.
THIRD GENERATION BETA-BLOCKERS: CARVEDILOL
Carvedilol is a nonspecific β1- and β2-receptor blocker with vasodilating α-receptor blocker properties and antioxidant effects.16 The α-adrenergic receptor blockade could lead to hypotension and dizziness, but also preserves cardiac output via afterload reduction of the left ventricle.
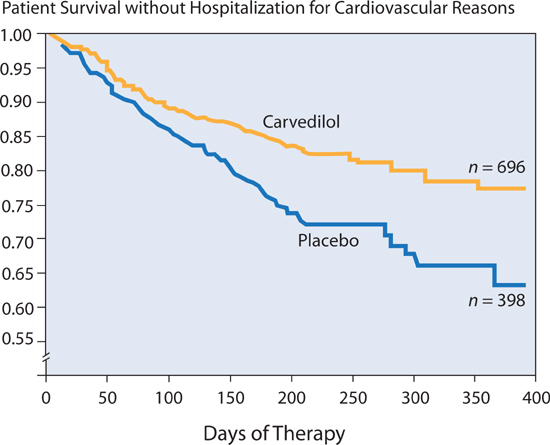
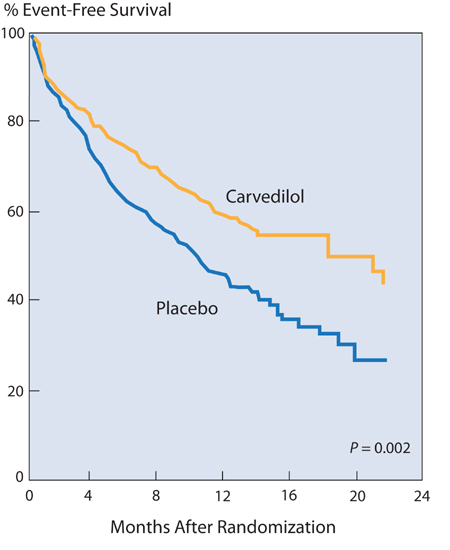
In the U.S. Carvedilol Heart Failure study,17 in patients with ejection fractions ≤ 35%, with a median duration of therapy of 6.5 months, there was a mortality of 7.8% in the placebo group and 3.2% in the carvedilol group—a 65% reduction in risk. The combined risk of death or cardiovascular hospitalization was reduced 38% (Figure 8.6).
FIGURE 8.6 The effect of carvedilol on rehospitalization and mortality in patients with chronic heart failure. Patients in the carvedilol group had a 38% lower risk of death or hospitalization for cardiovascular disease than patients in the placebo group (P < 0.001).17 Source: Adapted with permission from Packer et al., N Engl J Med. 1996;334(21):1349-1355.
Carvedilol in Severe Heart Failure: COPERNICUS Trial.
In the COPERNICUS trial, 2289 patients with an ejection fraction of < 25% and severe heart failure symptoms (NYHA class IV) without congestion, were randomized to receive placebo or carvedilol. After an average of 10.4 months of follow-up, carvedilol reduced the risk of cardiovascular death or hospitalization by 27% (P = 0.00002), in addition to reducing patient days in the hospital for any cause by 27% (P = 0.0005).18 Even in the highest-risk subset of patients, with recent or recurrent decompensation or an EF ≤ 15%, similar improvement was found (Figure 8.7).
FIGURE 8.7 Carvedilol for the highest-risk subgroup ( n = 624) of patients with severe heart failure (COPERNICUS trial). Compared to placebo, carvedilol significantly reduced the risk of death or cardiovascular hospitalization by 33% in patients with recent or recurrent heart failure decompensation or very depressed ejection fraction (≤ 15%). (P = 0.002)18 Source: Adapted with permission from Packer et al., Circulation. 2002;106(17):2194-2199.
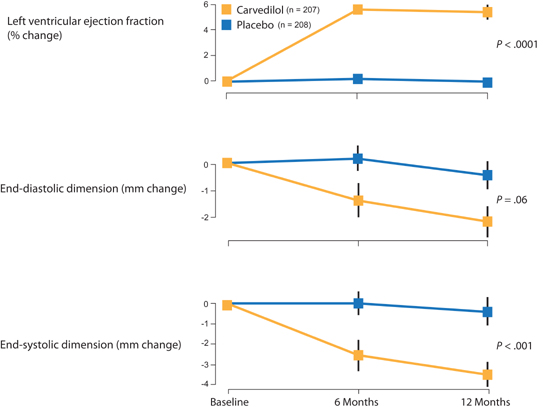
Effects of Carvedilol on Heart Size
Whereas ACE inhibitors can blunt increases in heart size following myocardial injury such as myocardial infarction, beta-blocker therapy might reverse myocardial enlargement.19 In patients with HF-rEF, carvedilol significantly increased ejection fraction, decreased end-systolic dimension, and showed a trend toward decreased end-diastolic dimension by echocardiography (Figure 8.8). This structural effect on the heart in patients with HF-rEF supports the hypothesis that chronic excessive catecholamine stimulation contributes to the adverse consequence of ventricular enlargement.
FIGURE 8.8 Carvedilol improves ejection fraction and reduces heart size in patients also on ACE inhibitor therapy.19 Source: Reprinted with permission from Lancet. 1997;349(9049):375-380.
INITIATING β-ADRENERGIC RECEPTOR BLOCKADE IN PATIENTS WITH HEART FAILURE: START LOW AND GO SLOW!
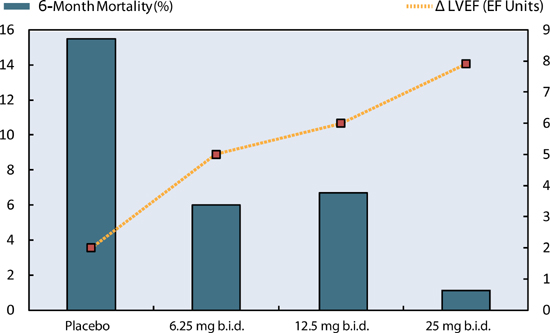
When starting beta-blockers in patients with heart failure, low doses should be used initially, then gradually titrated to higher target doses. This minimizes potential symptoms of dizziness, fatigue, or worsening heart failure during the initiation of therapy. Typically, if a patient is tolerating a beta-blocker, double the dose every 1 to 4 weeks until target doses are attained (e.g., carvedilol titrated from 3.125 to 25 mg twice daily over a series of steps). In the IMPACT-HF trial, patients who were started on beta-blockers during initial hospitalization for HF-rEF were more likely to still be taking them as outpatients 60 days later without increased side effects.20 Better outcomes can be achieved if target doses of beta-blockers can be reached (Figure 8.9). Carvedilol is also available in a sustained release preparation in a range of doses given once daily.21
FIGURE 8.9 Reaching target doses for carvedilol. With increasing doses of carvedilol, 6-month mortality decreased and left ventricular ejection fraction increased (Δ LVEF).22 Source: Adapted with permission Bristow et al., Circulation. 1996;94(11):2807-2816.
Potential Interactions with Other Therapies and Conditions
Diuretic dosage may require adjustment during the early phase of beta blockade. Dizziness, if due to hypotension, can improve with a reduction in diuretics. Alternatively, shortness of breath, if due to pulmonary congestion, may require an increase in diuretics.
Chronic obstructive lung disease is common in elderly patients with heart failure, and occasionally symptoms may be exacerbated by beta-blockers. However, in the absence of true hyperresponsive airway reactivity (asthma), carvedilol is usually well tolerated and the benefits of treatment are significant.23 Although leg claudication due to peripheral arterial disease may worsen with beta-blockers, this is uncommon with carvedilol.23 When patients develop intolerance of beta-blockers because of hypotension or worsening heart failure despite adjustment of other medications, this may portend clinical decline and transition from Stage C to Stage D heart failure.
OTHER BETA-BLOCKERS
The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF)24 found that compared to placebo, sustained-release metoprolol succinate, titrated to 200 mg per day, decreased mortality by 35% in patients with predominately NYHA class II and III heart failure and an ejection fraction ≤ 40% (Figure 8.10). When carvedilol was compared to immediate-release metoprolol tartrate, however, carvedilol was associated with an additional 14% decrease in risk of mortality.25 Thus, if metoprolol is used for HF-rEF, it should be the sustained-release, once-a-day formulation, metoprolol succinate.
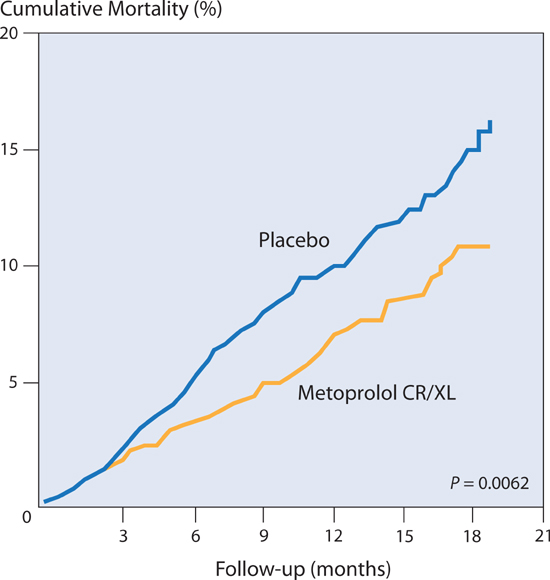
FIGURE 8.10 Beta-blocker effects on heart failure mortality. Metoprolol succinate compared to placebo in patients with chronic heart failure reduced the relative risk of mortality by 34%, n = 3991.24 Source: Adapted with permission from Lancet. 1999;353(9169):2001-2007.
In the Cardiac Insufficiency Bisoprolol Study (CIBIS II) trial in patients with either ischemic or nonischemic cardiomyopathy, a third beta-blocker, bisoprolol, was also associated with a 32% decrease in mortality compared to placebo.26
Mechanisms of Improvement with β-Adrenergic Receptor Blockade in Heart Failure
Beta-blockade can blunt the reduction in density and reduced sensitivity of β-adrenergic receptors seen with progressive heart failure.27 Furthermore, reversal of reduced myocardial gene expression of β-receptors and SERCA2a, and of fetal patterns of myosin expression (see Chapter 4) are associated with the beneficial clinical responses to beta-blocker therapy and improved left ventricular ejection fraction.28 The reduction in heart rate alone secondary to beta-blockade may improve myocardial energetics. This is supported by studies with ivabradine, a sinus node inhibitor (not available in the United States at this time), which slows heart rate by a non-adrenergic receptor mechanism. In the SHIFT trial in 6558 patients with an EF ≤ 35%, ivabradine reduced cardiovascular death or heart failure hospital admission by 18% when added to standard therapy in patients with resting heart rates of 70 bpm or higher.29
Mineralocorticoid Receptor Antagonists
Aldosterone contributes to the progression of heart failure including promotion of myocardial fibrosis.30 Medications known as mineralocorticoid receptor antagonists (MRAs) block aldosterone receptors. Although these medications are mild diuretics, MRAs also prevent hypokalemia associated with concomitant use of loop diuretics.31,32 Conversely, because the available MRA agents spironolactone and eplerenone can cause hyperkalemia or renal insufficiency, they should be withheld when serum creatinine is more than 2.5 mg/dL in men or more than 2.0 mg/dL in women (or estimated glomerular filtration rate < 30 mL/min/1.73 m2), and/or potassium > 5.0 mEq/L.14
SPIRONOLACTONE
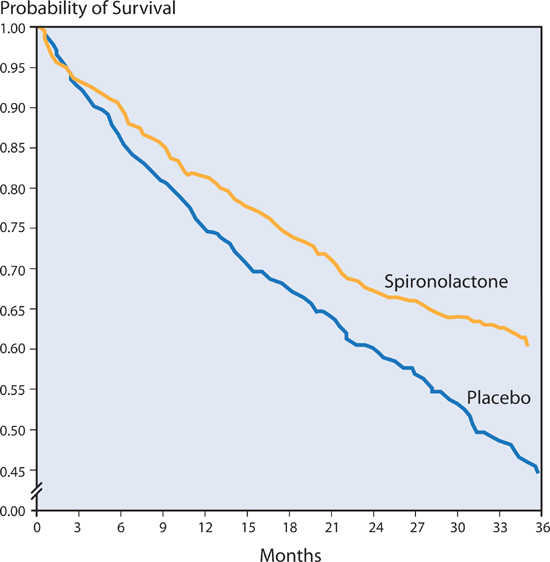
The RALES study randomized 1663 patients of NYHA class III or IV heart failure with left ventricular EF ≤ 35% to receive either 25 mg of spironolactone daily or placebo. After 2-years of follow up, spironolactone compared to the placebo group reduced the risk of death from any cause by 30%, and reduced the risk of hospitalization for heart failure by 35% (Figure 8.11).32 10% of men treated with spironolactone reported gynecomastia or breast tenderness, attributed to estrogen-like effects of spironolactone.
FIGURE 8.11 Spironolactone vs. placebo in patients with NYHA class III–IV heart failure. Note: Use of spironolactone resulted in a 30% decrease in risk of mortality (P < 0.001).32 Source: Adapted with permission from Pitt et al., N Engl J Med. 1999;341(10):709-717.
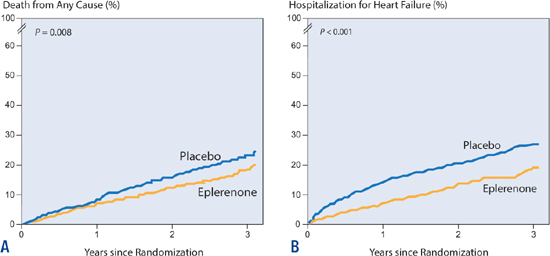
EPLERENONE
The EMPHASIS trial examined the MRA eplerenone in patients with less symptomatic HF-rEF. Zannad et al. randomly assigned 2737 patients with NYHA class II heart failure to receive either eplerenone or placebo. Eplerenone reduced the risk of the primary outcome (death from cardiovascular causes or a first hospitalization for heart failure) by 37% (P < 0.001). Secondary endpoints (death from any cause and hospitalization for heart failure) were also reduced with eplerenone (Figure 8.12).31 In contrast to spironolactone, eplerenone does not cause gynecomastia.
FIGURE 8.12 Eplerenone vs. placebo in patients with systolic heart failure and mild symptoms (NYHA class II). Eplerenone reduced risk of both death from any cause (Panel A) and hospitalization for heart failure (Panel B). (n = 2737; Death: HR 0.76, P = 0.008; Heart failure hospitalization: HR 0.58, P < 0.001).31 Source: Adapted with permission from Zannad et al., N Engl J Med. 2011;364(1):11-21.
Neutral Endopeptidase Inhibition
Extracellular neprilysin, a neutral endopeptidase enzyme (NEP), metabolizes vasoactive peptides including natriuretic peptides (e.g., ANP, BNP), bradykinin, and adrenomedullin. 33 NEP inhibition increases the levels of these substances and can offset the phenotype of progressive heart failure characterized by vasoconstriction, sodium retention, and maladaptive remodeling. In patients with HF-rEF in the PARADIGM-HF trial, a combined ARB (valsartan) and neprilysin inhibitor (sacubitril) led to a 21% further reduction in the primary composite endpoint of cardiovascular death and heart failure hospitalization compared to the ACE inhibitor enalapril.33 All-cause mortality was reduced 16%. Although NEP inhibition is not yet available, this class of medication might complement existing neurohormonal blocker therapies in the future, and when combined with an ARB provide a substitute for ACE inhibitor therapy.
Nitrates and Hydralazine
In the V-HeFT I trial, conducted prior to the routine use of ACE inhibitors or beta-blockers, the combination of the vasodilators hydralazine and isosorbide dinitrate reduced mortality, but not hospitalizations, in patients with heart failure already treated with digoxin and diuretics.34 Subsequently, a retrospective analysis found enhanced benefits of isosorbide dinitrate and hydralazine in the subset of African-American patients.35
In the African-American Heart Failure trial (A-HeFT),36 patients with NYHA class III and IV heart failure receiving background therapy (including ACE inhibitors or ARBs, beta-blockers, spironolactone, and diuretics), were assigned to placebo or the combination of oral isosorbide dinitrate (40 mg t.i.d.) and hydralazine (75 mg t.i.d.). Compared to the placebo group, with the addition of these therapies, overall risk of mortality was reduced by 43% (P = 0.01) and the rate of first hospitalization for heart failure was reduced 33% (P = 0.001). These results suggest that the combination of isosorbide dinitrate and hydralazine provides beneficial effects when added to current standard therapy in African-American patients (Figure 8.13).
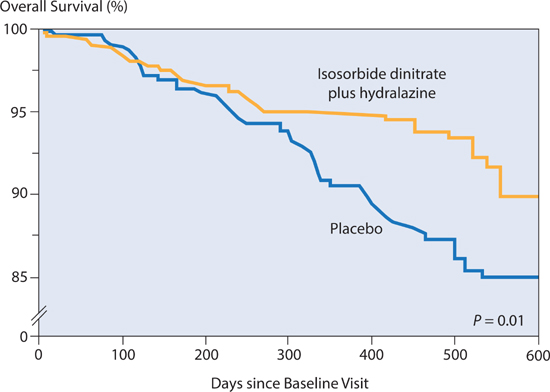
FIGURE 8.13 The administration of isosorbide dinitrate with hydralazine to African-American patients with NYHA class III–IV heart failure reduced the risk of death by 43%.36 Source: Adapted with permission from Taylor et al., N Engl J Med. 2004;351(20):2049-2057.
Digoxin
Controversy regarding the use of digitalis preparations has persisted over the last 200 years.37 Digitalis is a modest positive inotrope in patients with left ventricular dilation, slows the rate of conduction of atrial fibrillation at the AV node of the heart, and leads to a modest withdrawal of sympathetic tone, in part by a direct effect on the baroreceptors within the carotid sinus.37
The Digitalis Investigation Group (DIG) trial showed no difference in survival between patients with EF less than or equal to 45% randomized to digoxin versus placebo.38 The combined endpoints of death or hospitalization due to heart failure, however, were significantly reduced (Figure 8.14).
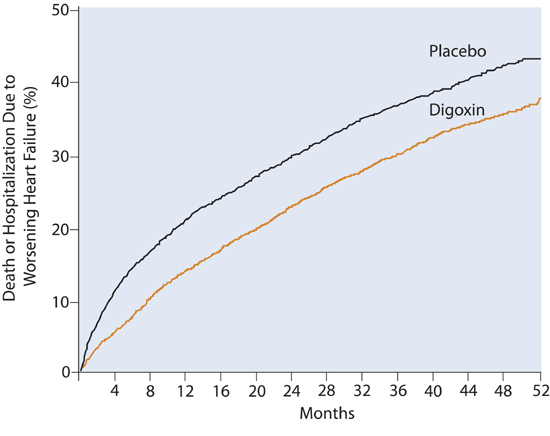
FIGURE 8.14 DIG Trial: Placebo vs. Digoxin. Incidence of death or hospitalization due to worsening heart failure comparing digoxin and placebo groups. (P < 0.001).38 Source: Adapted with permission from N Engl J Med. 1997;336(8):525-533.
DIGOXIN DOSING
Consider adding digoxin to patients in sinus rhythm with HF-rEF who remain symptomatic despite neurohormonal blockade and correction of volume overload. Because digoxin does not have an effect on overall mortality (only the combined endpoint of mortality and hospitalization), patients who are asymptomatic do not require digoxin. Optimal dosing is achieved when serum digoxin levels are ≤ 1.2 ng/mL typically at a dose of 0.125 mg daily in patients with normal renal function.39
Electrical Therapies for Heart Failure
Ventricular arrhythmias are a complication of structural heart disease, and electrical devices have impacted treatment strategies. An implantable cardioverter-defibrillator (ICD) is a pacemaker device with defibrillation capability that can electrically treat ventricular tachycardia and ventricular fibrillation. Cardiac resynchronization therapy (CRT) involves the placement of an additional lead, usually via the coronary sinus, onto the lateral wall of the left ventricle to improve left ventricular function in patients with congestive heart failure and prolonged QRS duration or pacemaker dependent bradycardia. A subcutaneous ICD (S-ICD) can treat ventricular arrhythmias without transvenous leads.
THE EVOLUTION OF DEVICE THERAPY FOR HEART FAILURE
Currently, device therapy complements pharmacologic treatment of HF-rEF, but this was not always the case. An ongoing partnership between cardiologists and biomedical engineers has produced effective, safe, small devices for evidence-based use (Figure 8.15).


FIGURE 8.15 Decreases in size of ICD between 1980 and 2014. Frontal (Panel A) and lateral (Panel B) views of Intec Systems (1980) and Mini ICD (2014). The original 1980 model was 300 g and 200 cm3 in volume; the 2014 model is 60 g and 26.5 cm3. Source: Intec Systems ICD courtesy of Dr. Ron Miller.
PREVENTION OF SUDDEN CARDIAC DEATH
Initially, ICDs were only placed in high-risk patients who had been resuscitated from an episode of sudden cardiac death or sustained ventricular arrhythmia. Subsequently, clinical trials demonstrated a survival benefit in patients with a low left ventricular ejection fraction without prior history of sudden cardiac death. The Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) randomized post-MI patients with persistent systolic dysfunction (LVEF ≤ 30%) to prophylactic ICD or conventional medical therapy.41 Overall, there was a 34% reduction in mortality risk in those who received a prophylactic ICD (Figure 8.16), attributed to a reduction in sudden cardiac death (3.8% vs. 10.0%; P < 0.01). In this trial, a higher rate of heart failure (20% vs. 15%) in a subset of patients in the ICD group was attributed to routine right ventricular pacing.40 In those programmed to minimal right ventricular pacing, the number of patients needed to treat to save 1 life was 6 (NNT = 6) based on data from 8 years of follow-up. This overall benefit occurred despite half of patients receiving their defibrillator through a higher-risk, transthoracic approach, compared to the current standard transvenous approach.
< div class='tao-gold-member'>