CHAPTER 5
Stage B: Asymptomatic Structural Heart Disease
“Before heart failure occurs, hypertrophy has generally become well developed and moreover it appears to have developed in a pattern unique to the inciting stress.”
—William Grossman, 19751
Who Is the Stage B Pre-Heart Failure Patient?
Stage B is composed of patients who have structural heart disease associated with risk of subsequent heart failure. By definition, the advancement from Stage A to Stage B heart failure can occur without recognition by patients especially if noncardiac factors limit physical activity. Thus, the clinical definition of the Stage B patient is often somewhat difficult to pinpoint. For example, the Mayo Clinic criteria include an assessment of physical activity (see complete criteria below).
STAGE B SYSTOLIC DYSFUNCTION
Stage B outcomes depend on the population studied and the criteria used to define structural heart disease. In patients with left ventricular systolic dysfunction (LVSD), it is estimated that there are four times as many asymptomatic (Stage B) as symptomatic Stage C and D patients combined.4
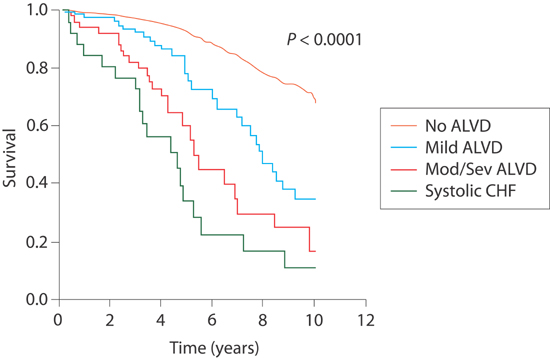
The Framingham Study assessed 4257 asymptomatic participants (age ≥ 40 years) and found an ejection fraction (EF) < 50% to be present in 6.0% of men and in 0.8% of women. As systolic function worsened, prognosis approached that of symptomatic systolic dysfunction (Figure 5.1).5
FIGURE 5.1 Survival curves in relationship to systolic dysfunction. Kaplan-Meier curves for survival (n = 4257). In the key, ALVD stands for asymptomatic left ventricular (systolic) dysfunction. No ALVD consists of subjects with normal left ventricular systolic function (EF > 50%) and no history of congestive heart failure (CHF). Mild ALVD indicates an EF of 40% to 50%. Mod/Sev ALVD indicates EF < 40%. Systolic CHF indicates symptomatic patients with systolic dysfunction.4 Source: Adapted with permission from Goldberg & Jessup, Circulation. 2006;113(24):2851-2860.
Subsequently, an analysis by Lam et al. in an elderly subset of the Framingham study population (mean age, 76 ± 5 years) assessed both LV systolic and diastolic dysfunction. Systolic dysfunction was defined as echocardiographic EF ≤ 45%, and diastolic dysfunction was defined by abnormal Doppler mitral inflow patterns showing abnormal relaxation, pseudonormal, or restrictive filling (see Chapter 7). Using these definitions, they found a prevalence of asymptomatic systolic dysfunction of 5% and diastolic dysfunction of 36%.6
STAGE B SYSTOLIC AND DIASTOLIC DYSFUNCTION
The Mayo Clinic sampled a cross-section of 2029 Olmsted County, Minnesota residents aged ≥ 45 years. When using clinical criteria including post-MI, systolic dysfunction, valvular heart disease, and left ventricular hypertrophy (LVH) either by ECG or on echocardiogram, they found a Stage B prevalence of 23%. When Doppler diastolic dysfunction (see Table 5.1) was included, the prevalence increased to 34% (Figure 5.2).3
TABLE 5.1 Mayo Clinic Definition Stage B Abnormal Echocardiogram.
Echocardiographic findings can be abnormal based on systolic or diastolic dysfunction. E/A ratio refers to transmitral Doppler velocity in early diastole (E) or during atrial inflow (A)3 (see also Chapter 7, Figure 7.2).
SYSTOLIC DYSFUNCTION: EF < 50% | |
DIASTOLIC DYSFUNCTION | E/A RATIO |
Normal | Normal E/A ratio |
Mild diastolic dysfunction | Decreased E/A ratio < 0.75 |
Moderate/“pseudonormal” diastolic dysfunction with other Doppler indices of elevated left ventricular end-diastolic filling pressure | E/A ratio 0.75 to 1.5 |
Severe diastolic dysfunction | Increased E/A ratio of > 1.5, deceleration time < 140 ms, Doppler indices of elevated left ventricular end-diastolic filling pressure |
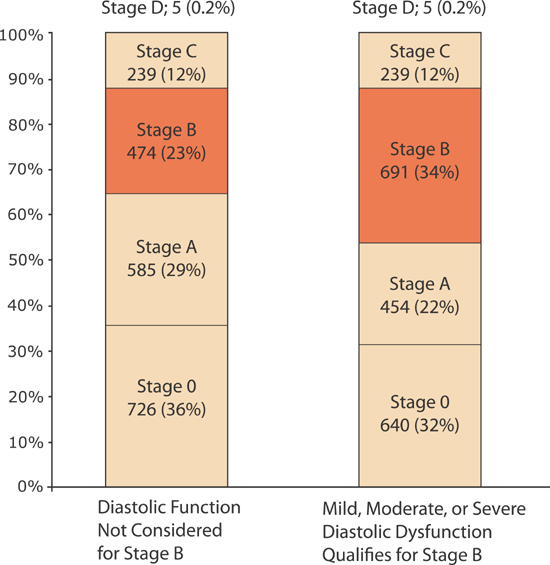
FIGURE 5.2 Prevalence of heart failure by stages with and without diastolic dysfunction as criteria for Stage B. All individuals were ≥ 45 years old. Stage 0 refers to the absence of any Stage A risk factors. Using the Mayo Clinic’s criteria, the inclusion of diastolic ventricular dysfunction (Table 5.1) as a qualifying abnormality for Stage B heart failure increases the prevalence of Stage B heart failure.3 Source: Adapted with permission from Ammar et al., Circulation. 2007;115(12):1563-1570.
Neurohumoral Continuum from Stage B to Stage C Systolic Dysfunction
The patient with structural heart disease can manifest a progressive increase in virtually all neurohumoral mediators.7 These activated systems can have acute circulatory and long-term gene expression effects in myocardial and vascular cells. High baseline levels of mediators can also blunt compensatory reserve for acute adjustments to circulatory stress.
NEUROHUMORAL ACTIVATION CAN PRECEDE SYMPTOMATIC HF-rEF
The Studies of Left Ventricular Dysfunction (SOLVD) trials evaluated normal subjects without heart disease (control group), patients with a left ventricular EF less than 35% prior to the development of heart failure symptoms (Stage B prevention group), and patients with recent development of heart failure symptoms (Stage C treatment group).8 Increasing levels of norepinephrine, plasma renin activity, and vasopressin were observed with increases in disease severity.
Norepinephrine
Plasma norepinephrine levels in Stage B patients with systolic dysfunction are elevated compared to normal controls but not as high as in patients with Stage C heart failure (Figure 5.3).
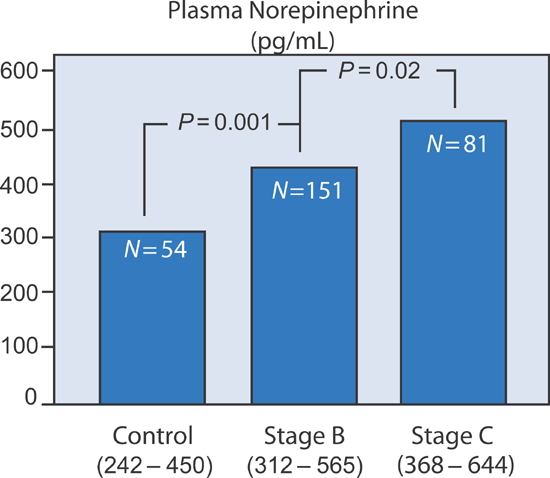
FIGURE 5.3 Plasma norepinephrine activity in SOLVD patient groups.7 Source: Adapted with permission from Francis et al., Circulation. 1990;82(5):1724-1729.
Plasma Renin Activity
Plasma renin activity, a mediator of angiotensin II formation, also increased within the three SOLVD groups (Figure 5.4).
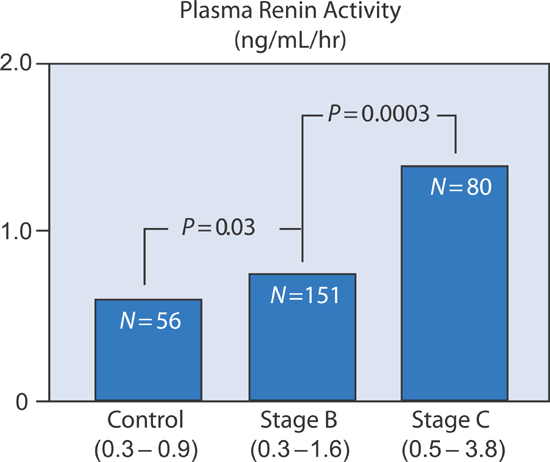
FIGURE 5.4 Plasma renin activity in SOLVD patient groups.7 Source: Adapted with permission from Francis et al., Circulation. 1990;82(5):1724-1729.
Vasopressin/Antidiuretic Hormone
Although pituitary vasopressin release is primarily stimulated by increases in plasma osmolality, it can also be triggered by baroreceptor activation with low cardiac output states and by angiotensin II (Figure 5.5). In advanced Stage C and Stage D patients, high levels of vasopressin can lead to hyponatremia (see Chapter 10).
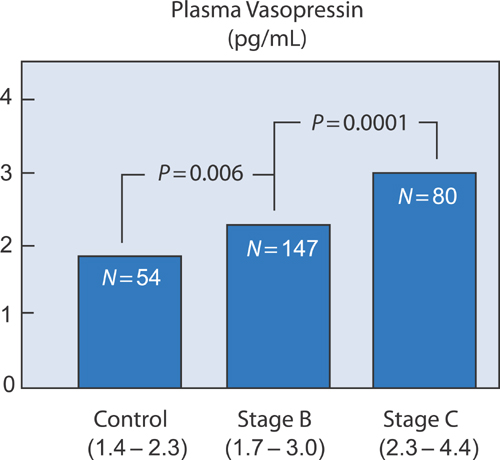
FIGURE 5.5 Plasma vasopressin/antidiuretic hormone activity in SOLVD patient groups.7 Source: Adapted with permission from Francis et al., Circulation. 1990;82(5):1724-1729.
The Continuum from Hypertension to HF-pEF
Similar to the pattern of neurohormonal activation, a continuum of diastolic dysfunction-associated abnormalities is seen when Doppler echocardiography assessments are made in a normal control group, in hypertensives with LVH identified by echocardiography but without heart failure symptoms, and in Stage C hypertensives with HF-pEF (Figure 5.6).
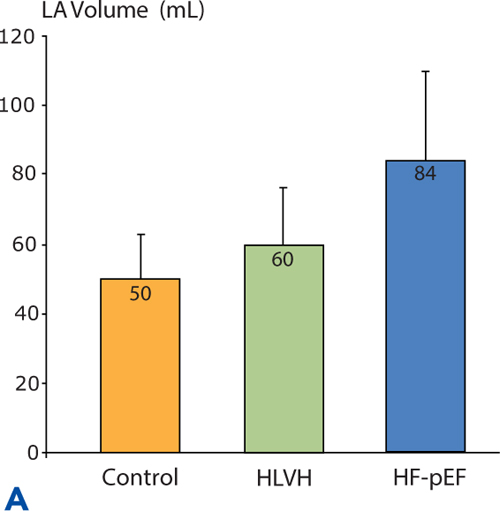
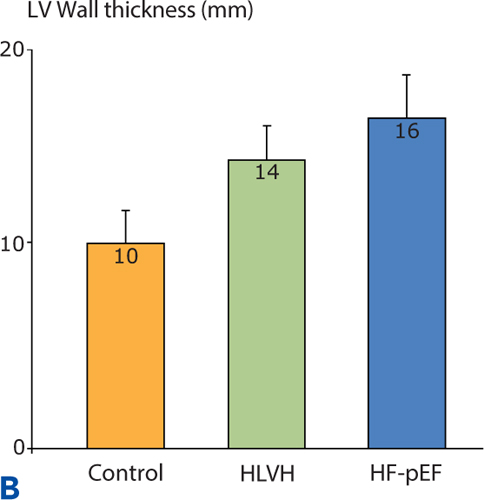
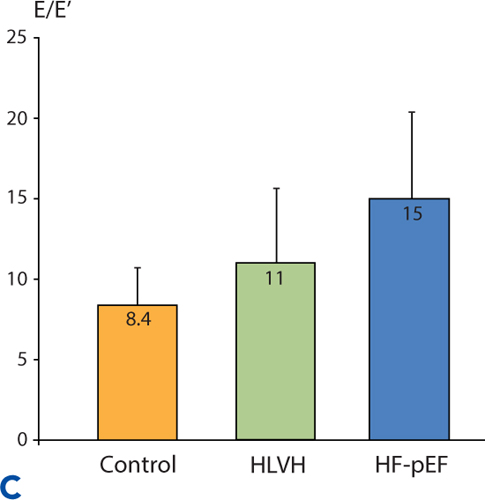
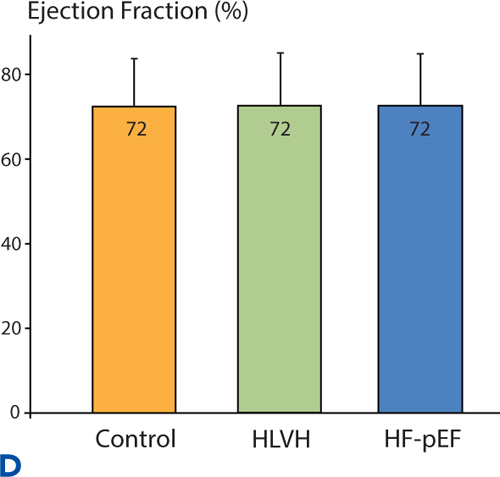
FIGURE 5.6 Continuum of echocardiographic abnormalities in patients with progressive stages of heart failure. Data were obtained in a cross-sectional study comparing normotensive control subjects without LVH (n = 56), hypertensive left ventricular hypertrophy (HLVH) subjects without heart failure (n = 40), and HF-pEF patients (n = 37). Diastolic dysfunction parameters in Panels A, B , and C are left atrial (LA) volume, left ventricular wall thickness, and E/E% respectively (see Chapter 7). Panel D shows EF% in the same study population. Overall EF% does not change even when diastolic dysfunction worsens.9 Source: Adapted with permission from Melenovsky et al., J Am Coll Cardiol. 2007;49(2):198-207.
HYPERTENSIVE LEFT VENTRICULAR HYPERTROPHY AS TARGET FOR THERAPY
Most classes of medications that lower high blood pressure are associated with regression of LVH by echocardiography that can be detected as early as 3 months after initiation of therapy.10 Whether left ventricular wall thickness or mass per se is justified as a target of antihypertensive therapy is uncertain.11 At present, blood pressure goals alone are most strongly correlated with improved patient outcomes, and the goal of regression of hypertrophy is not incorporated into therapeutic guidelines. Although hypertension is the most common cause of increased left ventricular wall thickness, many other causes exist (Tables 5.2, 5.3, and 5.4)
TABLE 5.2 Common causes of left ventricular hypertrophy.
CONDITION: | DIAGNOSTIC POINTERS |
Hypertension: 5%–10% due to secondary causes in adults | ECG: presence of LVH (prevalence ~30%) can predict prognosis Echo: concentric LVH MRI: may help identify aortic coarctation |
Aortic stenosis: • Ejection systolic murmur may be absent in low cardiac output state • Distinguish from dynamic LV outflow tract obstruction | ECG: LVH Echo: Low valve gradient and reduced valve area in patients with low ejection fraction |
Obesity: • BMI > 30 kg/m2 • Increased waist circumference | ECG: Attenuated ECG LVH due to body habitus (prevalence ~10%) |
Physiological LVH Athletic heart: • High-level endurance training • Resting bradycardia • LVH regression with deconditioning | ECG: LVH Echo: • Mild concentric LVH (rarely > 13 mm) • Volume-loaded (dilated) LV cavity • Preserved diastolic and long-axis function MRI: No late gadolinium enhancement |
Abbreviations: BMI, body mass index; ECG, electrocardiogram; Echo, echocardiogram; LV, left ventricle; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging.12 Source: Adapted from Yousef et al., Eur Heart J. 2013;34(11):802-808.
TABLE 5.3 Less common causes of left ventricular hypertrophy.
CONDITION: | DIAGNOSTIC POINTERS |
Sarcomere protein disease/hypertrophic cardiomyopathy: • Family history (population prevalence 1:500) • Leading cause of sudden death in young athletes • Risk stratification for sudden cardiac death | ECG: If LVH with anterior T-wave inversion, consider apical LVH Echo: • Asymmetrical septal hypertrophy common (but also can present with concentric or apical LVH, and right ventricular involvement) • Normal LV dimensions in early stages of disease • Systolic anterior motion of mitral valve, dilated left atrium, diastolic dysfunction, and dynamic LV outflow tract obstruction MRI: Intramyocardial late gadolinium enhancement Genetics: Autosomal dominant |
Amyloidosis : • Senile amyloid relatively common (20% in population over 80 years old) • Multisystem involvement with variable signs including: proteinuria, petechiae, peripheral and autonomic neuropathy, hepatosplenomegaly, macroglossia | ECG: Low voltage QRS, heart block, atrial fibrillation Echo: • LVH with preserved LV size and bi-atrial dilatation • Speckled LV septum • Restrictive physiology • Thickened interatrial septum and valve leaflets MRI: Subendocardial late gadolinium enhancement Other: Congo red staining of target organ biopsies |
Hemochromatosis: • Late presentation in females • Transfusion overload • Clinical constellation includes: bronze skin, arthritis, diabetes (and other endocrine abnormalities), and liver cirrhosis | ECG: LVH Echo: LVH with bi-ventricular and bi-atrial dilatation. Restrictive physiology MRI: Rapid signal decay (< 20 ms) on T2* imaging may suggest indication for venesection and/or iron chelation therapy Genetics: Human hemochromatosis protein (HFE) gene testing (autosomal recessive) |
Left ventricular noncompaction: • May have preserved or reduced ejection fraction • Warfarin may be useful for prevention of systemic embolism | Echo: Ratio of non-compacted to compacted myocardium > 2:1. Color flow Doppler demonstrates blood flow in deep inter-trabecular sinuses MRI: Tendency to over diagnose condition Genetics: Autosomal dominant in familial cases |
Abbreviations: ECG, electrocardiogram; Echo, echocardiogram; LV, left ventricle; LVH, left ventricular hypertrophy; MRI, magnetic resonance imaging.12 Source: Adapted with permission from Yousef et al., Eur Heart J. 2013;34(11):802-808.
TABLE 5.4 Uncommon genetic causes of left ventricular hypertrophy.
CONDITION: CLINICAL PEARLS AND FEATURES | DIAGNOSTIC POINTERS |
Fabry disease: • Deficiency of α-galactosidase A • Enzyme replacement therapy available | ECG: LVH, short P–R interval, heart block Laboratory: Proteinuria Genetics: Absence of male–male transmission due to X-linked inheritance. Female presentation later in life. |
Pompe disease: • Acid maltase deficiency • Limb-girdle and respiratory muscle weakness | ECG: LVH, short P–R interval, accessory pathways Laboratory: Serum CK elevated, no fasting hypoglycemia Echo: Concentric LVH with restrictive physiology Genetics: Autosomal recessive |
Danon disease: • Lysosomal-associated membrane protein 2 (LAMP2) deficiency • Skeletal muscle weakness, and mental retardation | ECG: LVH, short P–R interval, accessory pathways Laboratory: Reduced LAMP2 activity (a membrane protein assay) Echo: Concentric LVH with restrictive physiology Genetics: Autosomal recessive |
PRKAG2 cardiomyopathy: • Mutation of AMP-activated protein kinase γ2 gene | ECG: LVH, short P–R interval, accessory pathways Echo: Concentric LVH with restrictive physiology Genetics: Autosomal dominant |
Primary carnitine deficiency: • Functional carnitine transporter deficiency • Skeletal muscle weakness, hepatomegaly, abnormal fatty acid metabolism | ECG: LVH Echo: Concentric LVH with restrictive physiology Laboratory: Hypoglycemia, hyperammonemia, low plasma carnitine level Genetics: Autosomal recessive |
Mitochondrial: • Skeletal muscle weakness • Neurologic abnormalities | ECG: LVH Laboratory: Serum CK and glucose normal or elevated Echo: Concentric LVH with restrictive physiology Muscle biopsy: typical ragged red fibers Genetics: Mitochondrial DNA mutation analysis |
Source: Adapted with permission from Yousef et al., Eur Heart J. 2013;34(11):802-808.12
Cardiac and Noncardiac Interactions
Lam and coworkers evaluated the Framingham database for the interaction of cardiac and noncardiac organ dysfunction leading to the syndrome of heart failure. The coexistence of Stage B heart failure (either systolic or diastolic dysfunction) and anemia, renal, or pulmonary dysfunction favored the subsequent development of Stage C heart failure (Figure 5.7). In contrast, impaired liver function and white blood cell count did not affect the risk of subsequent heart failure.
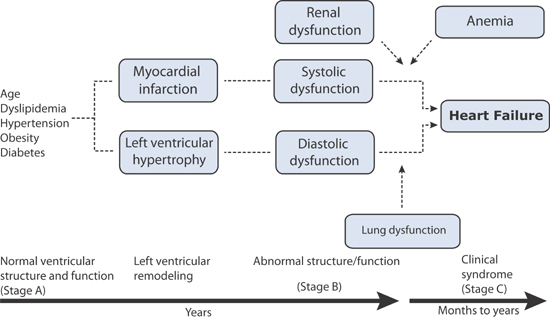
FIGURE 5.7 Interaction of cardiac and noncardiac dysfunctions and progression to heart failure.13 Source: Adapted with permission from Klein, Circulation. 2011;124(1):4-6.
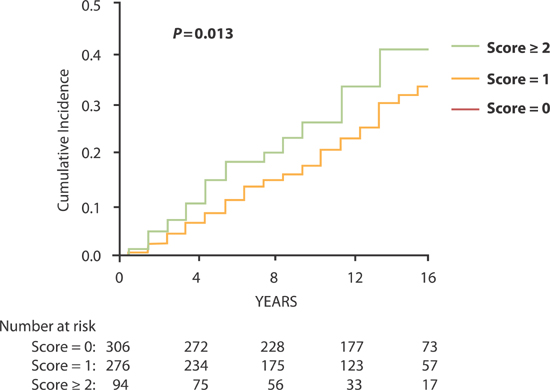
In a multivariate analysis, Stage C heart failure classification was separated into HF-rEF and HF-pEF and the antecedent factors determined. HF-rEF was associated with antecedent left ventricular systolic dysfunction, greater serum creatinine, and lower hemoglobin concentration; HF-pEF was associated with antecedent left ventricular diastolic dysfunction and lower ratio of forced expiratory volume in 1 second to forced expiratory volume (FEV1 : FVC) on pulmonary function testing.6 The combined impact of these indicators of noncardiac organ system dysfunction on the cumulative incidence of heart failure is shown in Figure 5.8.
FIGURE 5.8 Noncardiac Risk Score based on noncardiac major organ system dysfunction and cumulative incidence of symptomatic heart failure. Risk score ranges from 0 to 3. One point each was awarded for the presence of the following three parameters: serum creatinine > 1.05 mg/dL (92.8 µmol/L), FEV1 : FVC < 91% predicted, and hemoglobin concentration < 13 g/dL.6 Source: Adapted with permission from Lam et al., Circulation. 2011;124(1):24-30.
The presence of any 1 of the 3 significant noncardiac organ dysfunctions conferred a 30% increase in risk of subsequent development of symptomatic heart failure. When more than 1 dysfunction was present, risks were additive (Table 5.5 and Figure 5.8).6 Thus, the syndrome of heart failure represents more than the end result of all direct insults to the heart accumulated over time. It also reflects the contributions of vital noncardiac organs to circulatory homeostasis. Although more complex, this paradigm expands the scope of interventions beyond cardiovascular alone to include other organ systems that may potentially influence the development of heart failure in individuals at risk.
TABLE 5.5 Association of cardiac and noncardiac dysfunction with symptomatic heart failure.6 *Left ventricular systolic dysfunction defined as an EF < 50%. **Left ventricular diastolic dysfunction defined as heart failure and EF > 50%. † Noncardiac Risk Score (range 0–3). One point each for: serum creatinine > 1.05 mg/dL (92.8 µmol/L), FEV1 : FVC < 91% predicted, and hemoglobin concentration < 13 g/dL. CI = confidence interval.
CHARACTERISTICS | HAZARD RATIO | P |
ALL HEART FAILURE (HF-rEF + HF-pEF) | ||
LV systolic dysfunction* | 1.97 (1.05–3.68) | 0.034 |
LV diastolic dysfunction** | 1.40 (1.02–1.93) | 0.039 |
Noncardiac Risk Score† (incremental risk/1-unit increase) | 1.30 (1.06–1.60) | 0.013 |
HF-rEF | ||
LV systolic dysfunction* | 3.93 (1.86–8.30) | < 0.01 |
Serum creatinine > 1.05 mg/dL | 1.32 (1.04–1.69) | 0.025 |
Hemoglobin concentration/1-unit decrease | 1.31 (1.10–1.55) | 0.002 |
HF-pEF | ||
LV diastolic dysfunction** | 1.88 (1.13–3.13) | 0.016 |
FEV1 : FVC ratio < 91% predicted | 1.38 (1.04–1.83) | 0.024 |
< div class='tao-gold-member'>