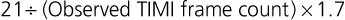Jessica L. Mega, David A. Morrow
ST-Elevation Myocardial Infarction
Management
The care of patients with ST-elevation myocardial infarction (STEMI) has transformed in conjunction with major shifts in the approach to reperfusion therapy from primarily pharmacologic to catheter-based strategies.1–4 With simultaneous advances in medical therapy, the case fatality rate for patients with STEMI has continued to decline.5 Nevertheless, optimal management of patients at high risk for or with established major complications of STEMI remains critical to the care of this condition. A discussion of the management of STEMI can follow the clinical course of the patient. Chapter 42 addresses primary and secondary prevention of coronary artery disease (CAD). This chapter deals with treatment at the time of onset of STEMI (prehospital issues, initial recognition and management in the emergency department, and reperfusion), hospital management (medications, complications, and preparation for discharge), and early secondary prevention after STEMI. Chapter 55 discusses percutaneous coronary intervention (PCI) in patients with STEMI. Chapter 36 describes the use of internal and external automated defibrillators for primary prevention of sudden cardiac death after myocardial infarction (MI).
Prehospital Management
Given the progressive loss of functioning myocytes with persistent occlusion of the infarct-related artery in STEMI (see Chapter 51), initial management aims to restore blood flow to the infarct zone as rapidly as possible. Primary PCI (see Chapter 55) is generally the preferred option, provided that an experienced operator and team can perform it in timely fashion.1,6,7 Missed opportunities for improvement in the care of STEMI include failure to deliver any form of reperfusion therapy in approximately 20% of patients and failure to minimize delays in reperfusion because of inefficient systems of care.5,8,9 The “chain of survival” for STEMI involves a highly integrated strategy beginning with patient education about the symptoms of MI (see Chapter 50) and early contact with the medical system, coordination of destination protocols in emergency medical service (EMS) systems, efficient practices in emergency departments to shorten door-to-reperfusion time, and expeditious implementation of the reperfusion strategy by a trained team.10,11 The American Heart Association (AHA) launched a national initiative to engineer improved health care delivery for STEMI, including implementation of systems that shorten total ischemic time (Tables 52-1 and 52-2) while emphasizing overall quality of care for STEMI.11,12
TABLE 52-1
Criteria for a System of Care for ST-Elevation Myocardial Infarction

Modified from www.americanheart.org/missionlifeline.
TABLE 52-2
Interventions to Improve Door-to-Device Times

From O’Gara PT, Kushner FG, Ascheim DD, et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78, 2013.
Prehospital Care
The prehospital care of patients suspected of having STEMI bears directly on the likelihood of survival. Most deaths associated with STEMI occur within the first hour of its onset and usually result from ventricular fibrillation (VF) (see Chapter 39). Hence immediate implementation of resuscitative efforts and rapid transportation of the patient to a hospital have prime importance. Major components of the delay from the onset of ischemic symptoms to reperfusion include the following1: (1) the time for the patient to recognize the seriousness of the problem and seek medical attention; (2) prehospital evaluation, treatment, and transportation; (3) the time for diagnostic measures and initiation of treatment in the hospital (e.g., “door-to-needle” time for patients receiving a fibrinolytic agent and “door-to-balloon” time for patients undergoing a catheter-based reperfusion strategy); and (4) the time from initiation of treatment to restoration of flow.
Patient-related factors that correlate with a longer time until deciding to seek medical attention include older age; female sex; black race; low socioeconomic status; low emotional or somatic awareness; history of angina, diabetes, or both; consulting a spouse or other relative; and consulting a physician.13,14 Health care professionals should heighten the level of awareness of patients at risk for STEMI (e.g., those with hypertension, diabetes, history of angina pectoris).1 They should use each patient encounter as a “teachable moment” to review and reinforce with patients and their families the need to seek urgent medical attention for a pattern of symptoms that includes chest discomfort, extreme fatigue, and dyspnea, especially if accompanied by diaphoresis or lightheadedness. Patients should also be instructed in the proper use of sublingual nitroglycerin and to call emergency services if the ischemic-type discomfort persists for more than 5 minutes.1
Emergency Medical Service Systems
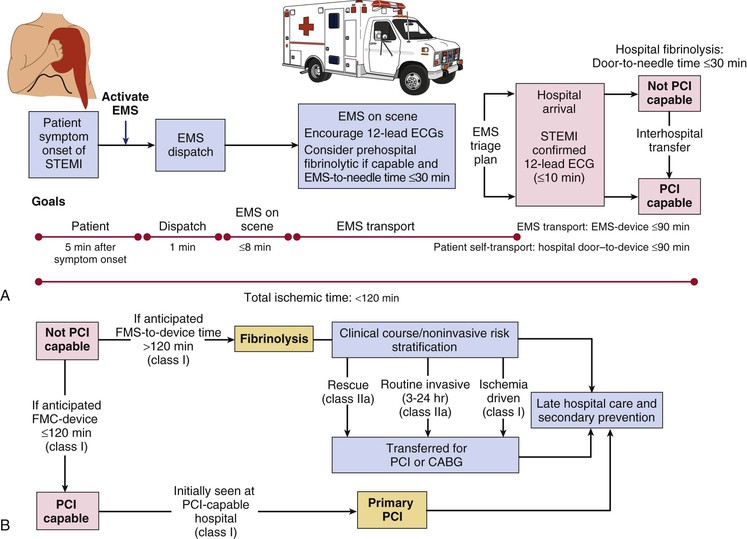
EMS systems have three major components: emergency medical dispatch, first response, and the EMS ambulance response. The expanded capability to record a prehospital 12-lead electrocardiogram (ECG) represents a major advance in EMS systems (Table 52-2).15 The ability to transmit such ECGs and to activate the STEMI care team before arrival at the hospital places EMS efforts at the center of the early response to STEMI.16,17 Ongoing efforts to shorten the time until treatment of patients with STEMI include improvement in the medical dispatch component by expanding 911 coverage, providing automated external defibrillators to first responders, placing automated external defibrillators in critical public locations, and greater coordination of the EMS ambulance response. Well-equipped ambulances and helicopters staffed by personnel trained in the acute care of patients with STEMI allow definitive therapy to begin during transport to the hospital (Table 52-3). Radiotelemetry systems that allow transmission of the electrocardiographic signal to a medical control officer are highly desirable for facilitating the triage of patients with STEMI and are becoming increasingly available in many communities (Fig. 52-1).
In addition to prompt defibrillation, the efficacy of prehospital care appears to depend on several factors, including early relief of pain with its deleterious physiologic sequelae, reduction of excessive activity of the autonomic nervous system, and treatment of arrhythmias such as ventricular tachycardia (VT)—but these efforts must not delay rapid transfer to the hospital (Fig. 52-1).
Prehospital Fibrinolysis
Multiple observational studies and several randomized trials have evaluated the potential benefits of prehospital versus in-hospital fibrinolysis.1,17 Although none of the individual trials showed a significant reduction in mortality with prehospital-initiated fibrinolytic therapy, earlier treatment generally provides greater benefit: a meta-analysis of all the available trials demonstrated a 17% reduction in mortality.1 The CAPTIM (Comparison of primary Angioplasty and Pre-hospital fibrinolysis In acute Myocardial infarction) trial, for example, reported a trend toward a lower mortality rate in patients with STEMI who received prehospital fibrinolysis than in those who received primary PCI, especially if they were treated within 2 hours of the onset of symptoms.1 Prehospital fibrinolysis is reasonable in settings in which substantial time can be saved by prehospital treatment because of long transportation times (i.e., 60 to 90 minutes or longer), physicians are present in the ambulance, or there is a well-organized EMS system with full-time paramedics who can obtain and transmit 12-lead electrocardiographic recordings from the field to an online medical command able to authorize prehospital fibrinolysis (Fig. 52-1).18
Management in the Emergency Department
When evaluating patients with chest pain in the emergency department, physicians must confront the difficult tasks of rapidly identifying patients who require urgent reperfusion therapy, triaging lower-risk patients to the appropriate setting within the hospital, and not discharging patients inappropriately while avoiding unnecessary admissions. A history of ischemic-type discomfort and the initial 12-lead ECG are the primary tools for screening patients with possible acute coronary syndromes (ACSs) for STEMI (see Chapter 50).19 Because the 12-lead ECG is at the center of the decision pathway for initiation of reperfusion therapy, it should be obtained promptly (≤10 minutes after hospital arrival) in patients with ischemic discomfort.1 More extensive use of prehospital 12-lead ECGs has also facilitated early triage of patients with STEMI.15 Because lethal arrhythmias can occur suddenly in patients with STEMI, all patients should have bedside monitoring of the ECG and intravenous access.
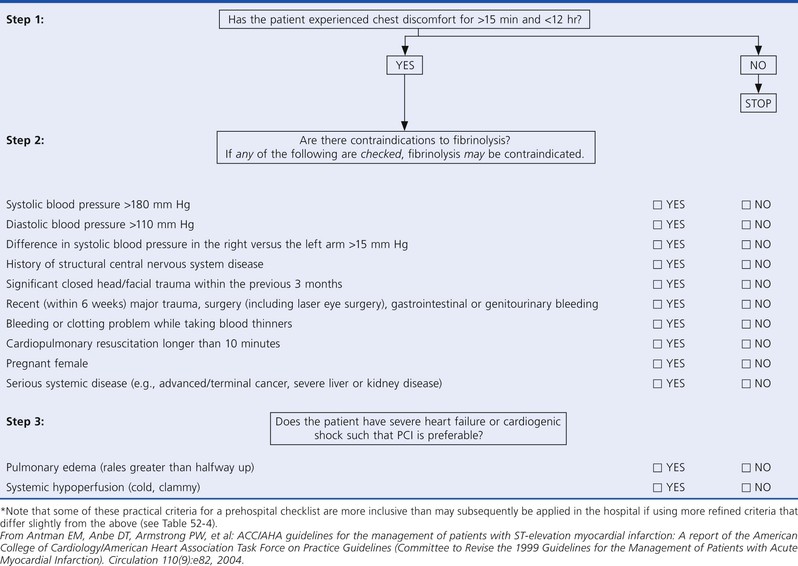
The presence of ST-segment elevation on the ECG in a patient with ischemic discomfort highly suggests thrombotic occlusion of an epicardial coronary artery, and it should trigger a well-rehearsed sequence of rapid assessment of the patient for initiation of a reperfusion strategy.1 If the initial ECG reveals ST-segment elevation of 0.1 mV or greater in at least two contiguous leads or a new or presumably new left bundle branch block, the patient should be evaluated immediately for a reperfusion strategy. Critical factors that weigh into selection of a reperfusion strategy include the time elapsed since the onset of symptoms, the risk associated with STEMI, the risk related to administering a fibrinolytic, and the time required to initiate an invasive strategy (Fig. 52-1). In non–PCI-capable hospitals, the initial assessment should include evaluation of the contraindications to administration of a fibrinolytic (Table 52-4). Patients with an initial ECG that reveals new or presumably new ST-segment depression and/or T wave inversion without ST-segment elevation are not considered candidates for immediate reperfusion therapy unless a posterior injury current is suspected (see Chapter 53).
TABLE 52-4
Contraindications to and Cautions in the Use of Fibrinolytics for Treating ST-Elevation Myocardial Infarction*

* Viewed as advisory for clinical decision making and may not be all-inclusive or definitive.
† Could be an absolute contraindication in low-risk patients with MI.
DBP = diastolic blood pressure; SBP = systolic blood pressure.
From O’Gara PT, Kushner FG, Ascheim DD, et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78, 2013.
Given the importance of time to reperfusion,7 emphasis has shifted to overall medical system goals, starting at the point of first medical contact with the patient.1,20 Benchmarks for medical systems to use when assessing the quality of their performance are a door-to-needle time of 30 minutes or less for initiation of fibrinolytic therapy and a door-to-device time of 90 minutes or less for percutaneous coronary perfusion (Fig. 52-1).1,4
In patients with a clinical history suggestive of STEMI (see Chapter 50) and an initial nondiagnostic ECG (i.e., no ST-segment deviation or T wave inversion), serial tracings should be obtained during evaluation in the emergency department. Emergency department staff can seek the sudden development of ST-segment elevation by periodic visual inspection of the bedside electrocardiographic monitor, by continuous ST-segment recording, or by auditory alarms when the ST-segment deviation exceeds programmed limits. Decision aids such as computer-based diagnostic algorithms, identification of high-risk clinical indicators, rapid determination of cardiac biomarkers, echocardiographic evaluation for regional wall motion abnormalities, and myocardial perfusion imaging have greatest clinical usefulness when the findings on the ECG are not diagnostic.
General Treatment Measures
Aspirin (See also Chapter 82)
Aspirin is effective across the entire ACS spectrum and is part of the initial management strategy for patients with suspected STEMI. Because low doses take several days to achieve a full antiplatelet effect, 162 to 325 mg should be administered at the first opportunity after initial medical contact.1 To achieve therapeutic blood levels rapidly, the patient should chew the tablet to promote buccal absorption rather than absorption through the gastric mucosa.21
Control of Cardiac Pain
Initial management of patients with STEMI should target relief of pain and its associated heightened sympathetic activity. Control of cardiac pain is typically achieved with a combination of analgesics (e.g., morphine) and interventions to favorably improve the balance of myocardial oxygen supply and demand, including oxygen, nitrates, and in appropriately selected patients, beta-adrenergic receptor–blocking agents (beta blockers).1
Analgesics.
Although a wide variety of analgesic agents—including meperidine, pentazocine, and morphine—have been used to treat the pain associated with STEMI, morphine remains the drug of choice, except in patients with well-documented morphine hypersensitivity. Doses of 4 to 8 mg administered intravenously and doses of 2 to 8 mg repeated at intervals of 5 to 15 minutes have been recommended1 until the pain is relieved or side effects emerge—hypotension, depression of respiration, or severe vomiting—that preclude further administration of the drug. Appropriate dosing of morphine sulfate will vary, however, depending on the patient’s age, body size, blood pressure, and heart rate.
Reduction of anxiety with successful analgesia diminishes the patient’s restlessness and the activity of the autonomic nervous system, with a consequent reduction in the heart’s metabolic demands. Morphine has beneficial effects in patients with pulmonary edema caused by peripheral arterial and venous dilation (particularly in those with excessive sympathoadrenal activity); it reduces the work of breathing and slows the heart rate secondary to combined withdrawal of sympathetic tone and augmentation of vagal tone. Observational studies have identified an association between the administration of morphine and adverse outcomes in patients with ACSs; however, it is challenging to disentangle this observation from confounding by indication.
Maintaining the patient in a supine position and elevating the lower extremities if blood pressure falls can minimize hypotension following the administration of nitroglycerin and morphine. Such positioning is undesirable in patients with pulmonary edema, but morphine rarely produces hypotension in these circumstances. Administration of atropine intravenously may be helpful in treating the excessive vagomimetic effects of morphine.
Nitrates.
By virtue of their ability to enhance coronary blood flow by coronary vasodilation and to decrease ventricular preload by increasing venous capacitance, sublingual nitrates are indicated for most patients with an ACS. At present, the only groups of patients with STEMI in whom sublingual nitroglycerin should not be given are those with suspected right ventricular infarction22 or marked hypotension (e.g., systolic pressure <90 mm Hg), especially if accompanied by bradycardia.
Once hypotension is excluded, a sublingual nitroglycerin tablet should be administered and the patient observed for improvement in symptoms or change in hemodynamics. If an initial dose is well tolerated and appears to be beneficial, further nitrates should be administered while monitoring vital signs. Even small doses can produce sudden hypotension and bradycardia, a reaction that can usually be reversed with intravenous atropine. Long-acting oral nitrate preparations should be avoided in the early course of STEMI because of the frequently changing hemodynamic status of the patient. In patients with a prolonged period of waxing and waning chest pain, intravenous nitroglycerin may help control the symptoms and correct the ischemia, but frequent monitoring of blood pressure is required. Initiation of a reperfusion strategy in patients with STEMI should not be delayed while assessing the patient’s response to sublingual or intravenous nitrates.
Beta-Adrenergic Blocking Agents.
These drugs aid in the relief of ischemic pain, reduce the need for analgesics in many patients, and reduce infarct size and life-threatening arrhythmias. Avoiding early intravenous blockade in patients with Killip class II or greater is important, however, because of the risk of precipitating cardiogenic shock.1 Routine use of intravenous beta blockers is no longer recommended in patients with STEMI, but administration of a beta blocker intravenously at the initial evaluation of patients with STEMI who are hypertensive and have ongoing ischemia is reasonable.1
A practical protocol for use of a beta blocker in this situation is as follows. (1) Exclude patients with heart failure, hypotension (systolic blood pressure <90 mm Hg), bradycardia (heart rate <60 beats/min), or significant atrioventricular (AV) block. (2) Administer metoprolol in three 5-mg intravenous boluses. (3) Observe the patient for 2 to 5 minutes after each bolus, and if the heart rate falls below 60 beats/min or systolic blood pressure falls below 100 mm Hg, do not administer any further drug. (4) If hemodynamic stability continues 15 minutes after the last intravenous dose, begin oral metoprolol tartrate, 25 to 50 mg every 6 hours for 2 to 3 days as tolerated, and then switch to 100 mg twice daily.1 Lower doses may be used in patients who have a partial decline in blood pressure with the initial dosing or who appear to be at higher risk (e.g., larger infarction) for the development of heart failure because of poor left ventricular performance. Infusion of an extremely short-acting beta blocker, such as esmolol, 50 to 250 mg/kg/min, may be useful in patients with relative contraindications to the administration of a beta blocker and in whom slowing of the heart rate is considered highly desirable.23
Oxygen.
Hypoxemia can occur in patients with STEMI and generally results from ventilation-perfusion abnormalities that are sequelae of left ventricular failure; concomitant intrinsic pulmonary disease may be an additional cause of hypoxemia. Treating all patients hospitalized for STEMI with oxygen for at least 24 to 48 hours is common practice based on the empiric assumption of hypoxia and evidence that increased oxygen in the inspired air may protect ischemic myocardium. However, augmentation of the fraction of oxygen in the inspired air does not elevate oxygen delivery significantly in patients who are not hypoxemic. Furthermore, it may increase systemic vascular resistance and arterial pressure and thereby lower cardiac output slightly.
In view of these considerations, arterial oxygen saturation can be estimated by pulse oximetry, and oxygen therapy can be omitted if the oximetric findings are normal. On the other hand, patients with STEMI and arterial hypoxemia should receive oxygen.1 In patients with severe pulmonary edema, endotracheal intubation and mechanical ventilation may be necessary to correct the hypoxemia and reduce the work of breathing.
Limitation of Infarct Size
Infarct size is an important determinant of prognosis in patients with STEMI. Patients who succumb from cardiogenic shock generally exhibit either a single massive infarct or a small to moderate infarct superimposed on multiple previous infarctions.24,25 Survivors with large infarcts frequently exhibit late impairment of ventricular function, and their long-term mortality rate is higher than that of survivors with small infarcts, in whom cardiac decompensation tends not to develop.26 In view of the prognostic importance of infarct size, the possibility of modifying infarct size has attracted much experimental and clinical attention (see Chapter 51, Fig. 51-11).7,27 Efforts to limit infarct size have been divided among several different (sometimes overlapping) approaches: (1) early reperfusion, (2) reduction of myocardial energy demands, (3) manipulation of energy production sources in the myocardium, and (4) prevention of reperfusion injury.
Dynamic Nature of Infarction
STEMI is a dynamic process that does not occur instantaneously but rather evolves over a period of hours. The fate of jeopardized, ischemic tissue can be favorably affected by interventions that restore myocardial perfusion, reduce microvascular damage in the infarct zone, decrease myocardial oxygen requirements, inhibit accumulation or facilitate washout of noxious metabolites, augment the availability of substrate for anaerobic metabolism, or blunt the effects of mediators of injury that compromise the structure and function of intracellular organelles and constituents of cell membranes. Strong evidence in experimental animals and suggestive evidence in patients indicate that ischemic preconditioning, a form of endogenous protection against STEMI, before sustained coronary occlusion decreases infarct size and is associated with a more favorable outcome along with decreased risk for extension of infarction and recurrent ischemic events. Brief episodes of ischemia in one coronary vascular bed may precondition myocardium in a remote zone and thereby attenuate the size of infarction in the latter when sustained coronary occlusion occurs.28
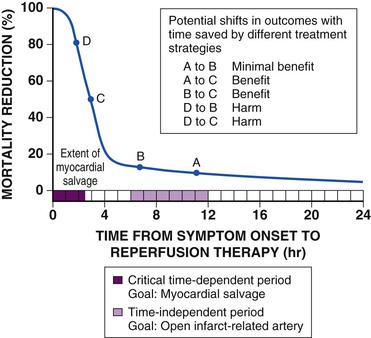
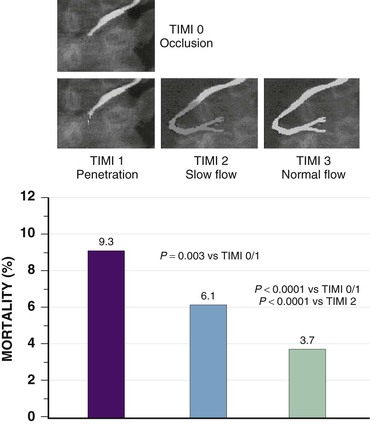
Perfusion of myocardium in the infarct zone appears to be reduced maximally immediately following coronary occlusion. Spontaneous recanalization of an occluded infarct-related artery occurs in up to a third of patients beginning at 12 to 24 hours. This delayed spontaneous reperfusion may enhance left ventricular function because it improves healing of infarcted tissue, prevents ventricular remodeling, and reperfuses hibernating myocardium. Yet, strategies involving pharmacologically induced and catheter-based reperfusion of the infarct vessel can maximize the amount of salvaged myocardium by accelerating the process of reperfusion and also implementing it in patients who would otherwise have an occluded infarct-related artery (Fig. 52-1) (see Chapter 55). An overarching concept that applies to all methods of reperfusion is the critical importance of time. Reduction of mortality in patients with STEMI is greatest the earlier the infarct artery is reperfused (Fig. 52-2).1
Additional factors that may limit infarct size during reperfusion include relief of coronary spasm, prevention of damage to the microvasculature, improved systemic hemodynamics (augmentation of coronary perfusion pressure and reduced left ventricular end-diastolic pressure), and collateral circulation. Prompt implementation of measures designed to protect ischemic myocardium and support myocardial perfusion may provide sufficient time for the development of compensatory mechanisms that limit the ultimate extent of infarction (see Chapter 51). Interventions designed to protect ischemic myocardium during the initial event may also reduce the extension of infarction or early reinfarction.
Routine Measures for Limitation of Infarct Size
Although timely reperfusion of ischemic myocardium is the most important technique for limiting infarct size, several routine measures to accomplish this goal apply to all patients with STEMI, regardless of whether they receive reperfusion therapy.1 The treatment strategies discussed in this section can be initiated at first medical contact and be continued throughout the hospital phase of care.
Myocardial oxygen consumption should be minimized by maintaining the patient at rest both physically and emotionally and by using mild sedation and a quiet atmosphere—in addition to the interventions already discussed. Administration of adrenergic agonists should be avoided whenever possible. All forms of tachyarrhythmia require prompt treatment because they increase myocardial oxygen needs. Heart failure should also be treated swiftly to minimize increases in adrenergic tone and hypoxemia (see the section Left Ventricular Failure).
If ongoing ischemia occurs, severe anemia should be corrected by the cautious administration of packed red blood cells, accompanied by a diuretic if there is any evidence of left ventricular failure. Associated conditions, particularly infections and accompanying tachycardia, fever, and elevated myocardial oxygen needs, require management.
Reperfusion Therapy
General Concepts
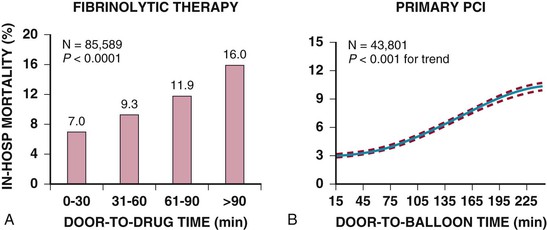
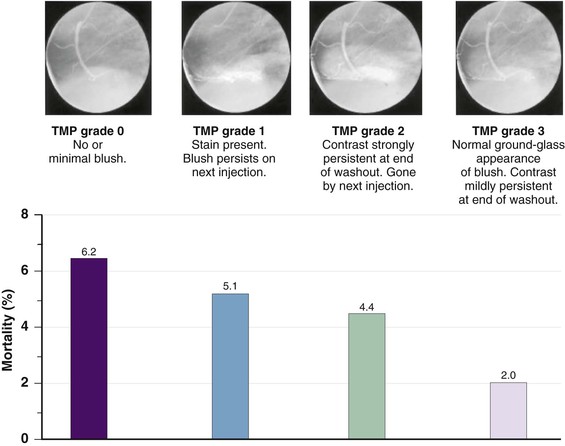
Although late spontaneous reperfusion occurs in some patients, thrombotic occlusion persists in most patients with STEMI. Timely reperfusion of jeopardized myocardium is the most effective way of restoring the balance between myocardial oxygen supply and demand.29 The dependence of myocardial salvage on the time elapsed until treatment pertains to patients treated with either fibrinolysis or PCI1,30,31 (Fig. 52-3; also see Fig. 52-2). The efficacy of fibrinolytic agents decreases as coronary thrombi mature over time (Fig. 52-3). Analyses adjusted for baseline risk, however, demonstrate a statistically significant increase in in-hospital and long-term mortality with progressive delays between the onset of symptoms and PCI.30,32 Each 30-minute delay from symptom onset to PCI increases the relative risk (RR) for 1-year mortality by 8%.1
In some patients, particularly those with cardiogenic shock, tissue damage occurs in a “stuttering” manner rather than abruptly. This concept of the infarction process, as well as the observation that the incidence of complications of STEMI in both the early and late postinfarction periods depends on infarct size, underscores the need for careful history taking to ascertain whether the patient appears to have had repetitive cycles of spontaneous reperfusion and reocclusion. Determining the precise time of onset of the infarction process in these patients, however, can be difficult and sometimes misleading. In such patients with waxing and waning ischemic discomfort, a rigid time interval from the first episode of pain should not be used when determining whether a patient is “outside the window” for benefit from acute reperfusion therapy.
Pathophysiology of Myocardial Reperfusion
Prevention of cell death by restoration of blood flow depends on the severity and duration of the preexisting ischemia. Substantial experimental and clinical evidence indicates that the earlier blood flow is restored, the more favorably influenced are recovery of left ventricular systolic function, improvement in diastolic function, and reduction in overall mortality.1 Collateral coronary vessels also appear to influence left ventricular function following reperfusion.33 They provide sufficient perfusion of myocardium to slow cell death and probably have greater importance in patients undergoing reperfusion later than 1 to 2 hours after coronary occlusion. Even after successful reperfusion and despite the absence of irreversible myocardial damage, a period of postischemic contractile dysfunction can occur—a phenomenon referred to as myocardial stunning.34
Reperfusion Injury
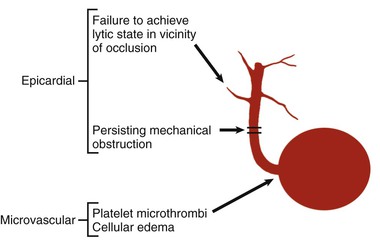
The process of reperfusion, although beneficial in terms of myocardial salvage, may be accompanied by adverse sequelae described by the term reperfusion injury (see Chapter 51).35,36 Several types of reperfusion injury occur in experimental animals: (1) lethal reperfusion injury, which refers to reperfusion-induced death of cells that were still viable at the time of restoration of coronary blood flow; (2) vascular reperfusion injury, which is progressive damage to the microvasculature such that there is an expanding area of no-reflow and loss of coronary vasodilatory reserve; (3) stunned myocardium, in which salvaged myocytes display a prolonged period of contractile dysfunction following restoration of blood flow because of abnormalities in intracellular metabolism leading to reduced energy production; and (4) reperfusion arrhythmias, which refers to bursts of VT (and on occasion, VF) that occur within seconds of reperfusion.37 Evidence suggests that vascular reperfusion injury, stunning, and reperfusion arrhythmias can all occur in patients with STEMI. The concept of lethal reperfusion injury to potentially salvageable myocardium remains controversial, both in animals and in humans.36,38,39
Microvasculature damage in the reperfused myocardium can lead to a hemorrhagic infarct (see Chapter 51). Fibrinolytic therapy appears more likely than catheter-based reperfusion to produce hemorrhagic infarction. Although concern has been raised that this hemorrhage may lead to extension of the infarct, such does not appear to be the case. Histologic study of patients not surviving despite successful reperfusion has revealed hemorrhagic infarcts, but this hemorrhage does not usually extend beyond the area of necrosis.
Protection Against Reperfusion Injury
A variety of adjunctive therapies have been proposed to mitigate the injury that occurs after reperfusion, including preservation of microvascular integrity by using antiplatelet agents and antithrombins to minimize embolization of atheroembolic debris, prevention of inflammatory damage, and metabolic support of the ischemic myocardium.36,39,40 The effectiveness of interventions directed against reperfusion injury appears to decline rapidly the later that they are administered after reperfusion. In animal models, no beneficial effect is detectable after 45 to 60 minutes of reperfusion has elapsed. Intriguingly, the phenomenon of induction of transient ischemia in other vascular beds has also been associated with a reduction in reperfusion injury, a concept called remote conditioning.28
An alternative experimental approach to protection against reperfusion injury is called postconditioning, which involves introducing brief, repetitive episodes of ischemia alternating with reperfusion.41 This appears to activate the cellular protective mechanisms centering around prosurvival kinases.42 Many of these protective kinases are also activated during ischemic preconditioning. Clinical studies in patients with STEMI undergoing PCI have provided evidence that postconditioning protects the human heart and is associated with reduced infarct size and improvement in myocardial perfusion.43
Reperfusion Arrhythmias
Transient sinus bradycardia occurs in many patients with inferior infarcts at the time of acute reperfusion, often accompanied by some degree of hypotension. This combination of hypotension and bradycardia with a sudden increase in coronary flow may involve activation of the Bezold-Jarisch reflex.44 Premature ventricular contractions, accelerated idioventricular rhythm, and nonsustained VT also commonly follow successful reperfusion. Although some investigators have postulated that early afterdepolarizations participate in the genesis of reperfusion-related ventricular arrhythmias, they are present during both ischemia and reperfusion and therefore not likely to be involved in the development of reperfusion-associated VT or VF.
When present, rhythm disturbances may actually indicate successful restoration of coronary flow, but their specificity for successful reperfusion is limited. In general, clinical features are inaccurate markers of reperfusion, with no single clinical finding or constellation of findings being reliably predictive of angiographically demonstrated coronary artery patency.1
Although reperfusion arrhythmias may show a temporal clustering at the time of restoration of coronary blood flow in patients after successful fibrinolysis, this brief “electrical storm” is generally innocuous, and therefore no prophylactic antiarrhythmic therapy is necessary and specific treatment is not indicated, except in rare cases of symptomatic or hemodynamically significant reperfusion arrhythmias.1
Late Establishment of Patency of the Infarct Vessel
The improved survival and ventricular function after successful reperfusion may not result entirely from limitation of infarct size.45 Poorly contracting or noncontracting myocardium in a zone that is supplied by a stenosed infarct-related artery with slow anterograde perfusion may still contain viable myocytes. The function of so-called hibernating myocardium can be improved by PCI to augment flow in the infarct-related artery.46,47
Summary of the Effects of Myocardial Reperfusion
Disruption of plaque in the culprit vessel and subsequent thrombus formation produces complete occlusion of the infarct-related coronary artery. STEMI occurs with the ensuing development of left ventricular dilation and ultimately cell death through a combination of pump failure and electrical instability (see Chapter 51). Early reperfusion shortens the duration of coronary occlusion, minimizes the degree of ultimate left ventricular dysfunction and dilation, and reduces the probability that pump failure or malignant ventricular tachyarrhythmias will develop in patients with STEMI. Late reperfusion of stenosed infarct arteries may also restore contractile function in hibernating myocardium.
Fibrinolysis
Fibrinolysis recanalizes the thrombotic occlusion associated with STEMI, and restoration of coronary flow reduces infarct size and improves myocardial function and survival over both the short and long term.48 Patients treated within the first 1 to 2 hours after the onset of symptoms seem to have the greatest potential for long-term improvement in survival with fibrinolysis.1
Effect of Fibrinolytic Therapy on Mortality
Early intravenous fibrinolysis improves survival in patients with STEMI.1 The benefit of fibrinolytic therapy appears to be greatest when agents are administered as early as possible, with the most dramatic results occurring when the drug is given less than 1 to 2 hours after symptoms begin.2
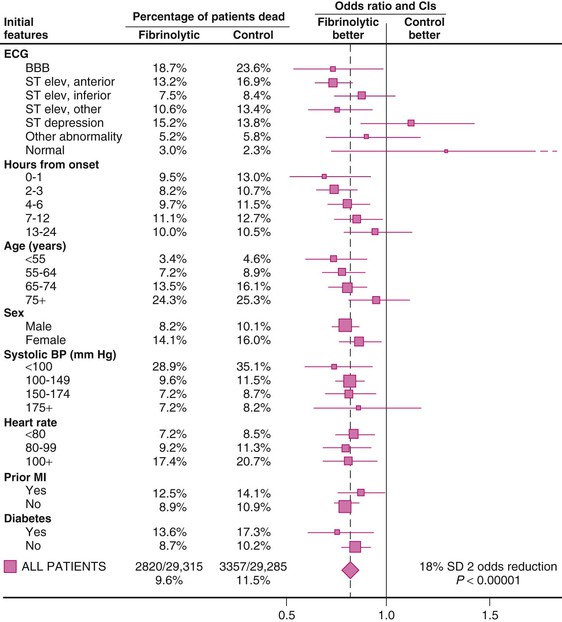
The Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group performed a comprehensive overview of nine trials of thrombolytic therapy, each of which enrolled more than 1000 patients. Absolute mortality rates for the control and fibrinolytic groups stratified by initial features are shown in Figure 52-7. The overall results indicated an 18% reduction in short-term mortality, but as much as a 25% reduction in mortality in the subset of 45,000 patients with ST-segment elevation or bundle branch block. Two trials, LATE (Late Assessment of Thrombolytic Efficacy) and EMERAS (Estudio Multicéntrico Estreptoquinasa Repúblicas de América del Sur), when viewed together, provide evidence that a reduction in mortality may still be observed in patients treated with thrombolytic agents between 6 and 12 hours after the onset of ischemic symptoms. Data from the LATE and EMERAS trials and the FTT overview form the basis for extending the window of treatment with fibrinolytics up to 12 hours after the onset of symptoms. As cited in the American College of Cardiology Foundation (ACCF)/AHA guidelines for the management of ST-elevation MI (referred to hereafter as the guidelines), Boersma and colleagues pooled the trials in the FTT overview, two smaller studies with data on time until randomization, and 11 additional trials.1 Patients were divided into six time categories from the onset of symptoms to randomization. A nonlinear relationship of treatment benefit to time was observed, with the greatest benefit occurring in the first 1 to 2 hours after the onset of symptoms.1
The mortality effect of fibrinolytic therapy in elderly patients is of considerable interest and controversy. Although patients older than 75 years were initially excluded from randomized trials of fibrinolytic therapy, they now constitute approximately 15% of those studied in trials of fibrinolysis and approximately 35% of those analyzed in registries of patients with STEMI.61 Barriers to initiation of therapy in older patients with STEMI include a protracted period of delay in seeking medical care, a lower incidence of ischemic discomfort and greater incidence of atypical symptoms and concomitant illnesses, and an increased incidence of nondiagnostic findings on the ECG.61 Younger patients with STEMI achieve a slightly greater relative reduction in mortality than elderly patients do, but the higher absolute mortality in elderly patients results in similar absolute reductions in mortality.62
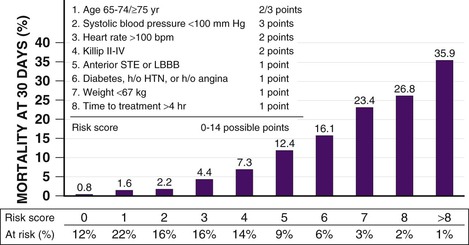
Several models have integrated the many clinical variables that affect a patient’s risk for mortality before the administration of fibrinolytic therapy. A convenient, simple, bedside risk-scoring system for predicting 30-day mortality at initial evaluation of fibrinolytic-eligible patients with STEMI was developed by Morrow by using the InTIME-II trial database (Fig. 52-8).63 Modeling of mortality risk cannot cover all clinical scenarios, however, and should supplement clinical judgment in individual cases. For example, patients with inferior STEMI who might otherwise be considered to have a low risk for mortality and for whom many physicians have questioned the benefits of fibrinolytic therapy might be in a higher mortality risk subgroup if their inferior infarction is associated with right ventricular infarction, precordial ST-segment depression, or ST-segment elevation in the lateral precordial leads.
The short-term survival benefit enjoyed by patients who receive fibrinolytic therapy is maintained over the 1- to 10-year follow-up. Room for improvement remains. Advances in adjunctive antiplatelet and antithrombin therapies have led to reductions in the rate of reinfarction after fibrinolysis for STEMI.2,48
Comparison of Fibrinolytic Agents (See Chapter 82)
Comparative features of the approved fibrinolytic agents for intravenous therapy are presented in Table 52-5. All fibrinolytic agents exert their effect by converting the proenzyme plasminogen to the active enzyme plasmin. The so-called fibrin-specific fibrinolytics are those that are relatively inactive in the absence of fibrin but in its presence substantially increase their activity on plasminogen.
TABLE 52-5
Comparison of Approved Fibrinolytic Agents
| FIBRINOLYTIC AGENT | DOSE | FIBRIN SPECIFICITY* | FIBRINOGEN DEPLETION | ANTIGENIC | PATENCY RATE (90-min TIMI 2 OR 3 FLOW) |
| Fibrin Specific | |||||
| Tenecteplase (TNK) | Single IV weight-based bolus† | ++++ | Minimal | No | 85% |
| Reteplase (r-PA) | 10 units + 10-unit IV boluses given 30 min apart | ++ | Moderate | No | 84% |
| Alteplase (t-PA) | 90-min weight-based infusion‡ | ++ | Mild | No | 73-84% |
| Non–Fibrin Specific | |||||
| Streptokinase§ | 1.5 million units IV given over 30-60 min | No | Marked | Yes‖ | 60-68% |
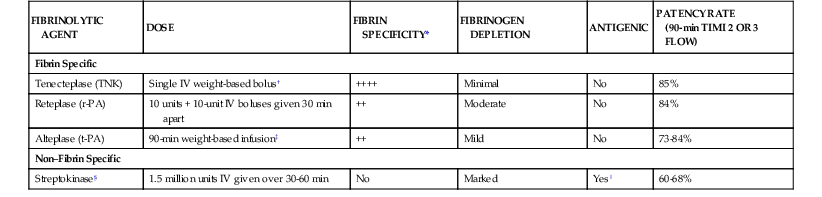
* Strength of fibrin specificity: ++++ is stronger; ++ is less strong.
† Bolus of 30 mg for weight less than 60 kg, 35 mg for 60 to 69 kg, 40 mg for 70 to 79 kg, 45 mg for 80 to 89 kg, and 50 mg for 90 kg or greater.
‡ Bolus of 15 mg, infusion of 0.75 mg/kg for 30 minutes (maximum, 50 mg), then 0.5 mg/kg (maximum, 35 mg) over the next 60 minutes; the total dose not to exceed 100 mg.
§ Streptokinase is no longer marketed in the United States but is available in other countries.
‖ Streptokinase is highly antigenic and absolutely contraindicated within 6 months of previous exposure because of the potential for serious allergic reaction.
r-PA = reteplase plasminogen activator; t-PA = tissue plasminogen activator.
From O’Gara PT, Kushner FG, Ascheim DD, et al: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 61:e78, 2013.
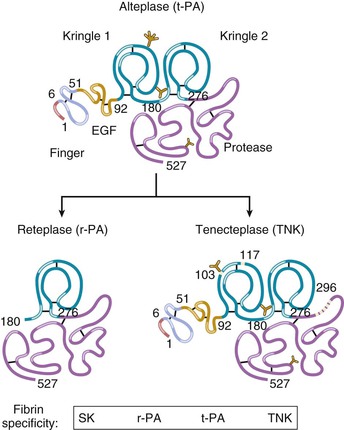
The tissue plasminogen activator (t-PA) molecule contains five domains (Fig. 52-9).64 In the absence of fibrin, t-PA is a weak plasminogen activator; fibrin provides a scaffold on which t-PA and plasminogen are held in such a way that the catalytic efficiency of plasminogen activating t-PA is increased many-fold. A dose regimen of t-PA administered over a 90-minute period produces more rapid thrombolysis than does a 3-hour fixed-rate infusion. Therefore the recommended dosage for t-PA is the 90-minute “accelerated” regimen.
Modifications in the native t-PA structure have yielded a group of fibrinolytic agents with prolonged plasma clearance that allows them to be administered as a bolus (Fig. 52-9 and Table 52-5) rather than as the bolus and infusion by which accelerated-dose t-PA is administered.64 Reteplase (double fixed-dose bolus) and tenecteplase (single weight-based bolus) have both been compared with accelerated t-PA. Both these newer agents were associated with mortality rates similar to that achieved with accelerated t-PA, but with more convenient dosing. In one large trial, tenecteplase was found to have a lower rate of major bleeding than accelerated t-PA did.
Other Fibrinolytic Agents
Streptokinase, a protein secreted by several species of streptococci, binds and activates human plasminogen and is an inexpensive and effective fibrinolytic agent that is still used in some regions of the world. Urokinase is used for STEMI on rare occasions as an intracoronary infusion.
Effect on Left Ventricular Function
As with survival, improvement in global left ventricular function is related to the time of initiation of fibrinolytic treatment, with the greatest improvement occurring with the earliest therapy.2 Although precise measurements of infarct size would be an ideal endpoint for clinical reperfusion studies, such measures have proved impractical. Attempts to use the left ventricular ejection fraction as a surrogate for infarct size have not been productive because little difference is seen in the ejection fraction between treatment groups that show a significant difference in mortality. Methods of assessing left ventricular function, such as end-systolic volume or quantitative echocardiography, are more revealing because patients with smaller volumes and better-preserved ventricular shape have improved survival. The myocardial salvage index, defined as the difference between the initial perfusion defect (e.g., by sestamibi scintigraphy) and the final perfusion defect, is a useful means for comparing the effectiveness of reperfusion therapies.65 Characterization of left ventricular volumes concurrently with the extent of scar as revealed by myocardial delayed enhancement, as well as ischemia with adenosine stress perfusion and cardiac MRI, provides significant incremental prognostic information over other clinical variables and is an emerging strategy for risk stratification after STEMI.66–68
Complications of Fibrinolytic Therapy
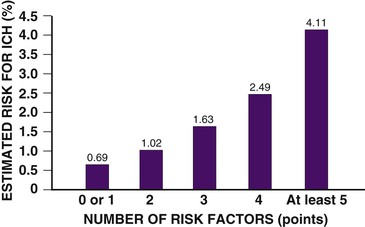
Bleeding complications are most common, and intracranial hemorrhage is the most serious complication of fibrinolytic therapy; its frequency is generally less than 1% but varies with the clinical characteristics of the patient and the fibrinolytic agent used (Fig. 52-10).1 Intracranial bleeding in the setting of fibrinolysis for STEMI is associated with a high case fatality rate. Nonintracranial bleeding can also result in increased morbidity, but whether it is causal of higher overall mortality, after taking into account the higher-risk clinical characteristics that also predispose patients to bleeding during treatment of STEMI, is uncertain.69,70