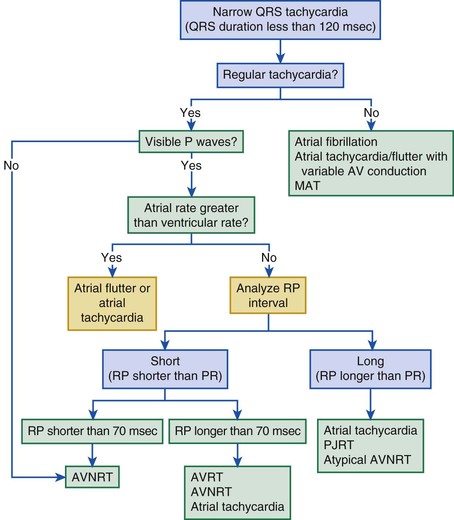
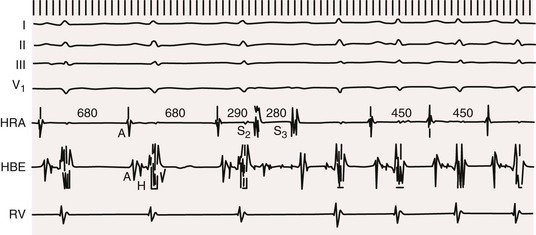
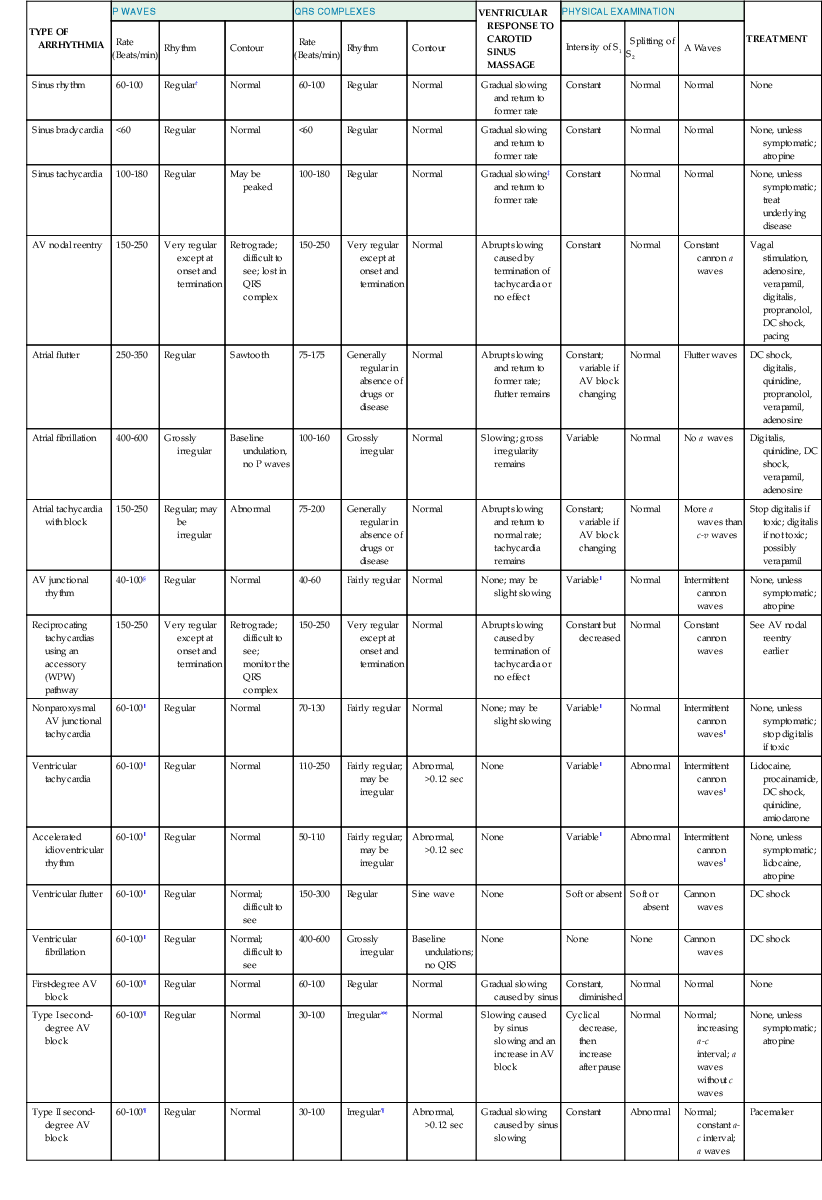
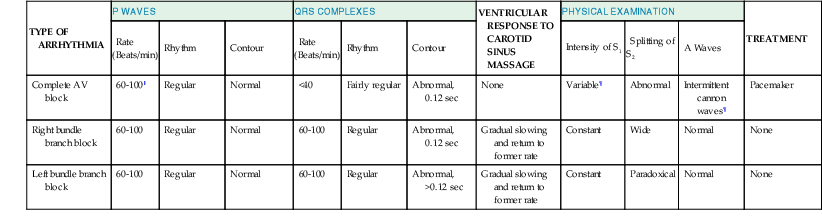
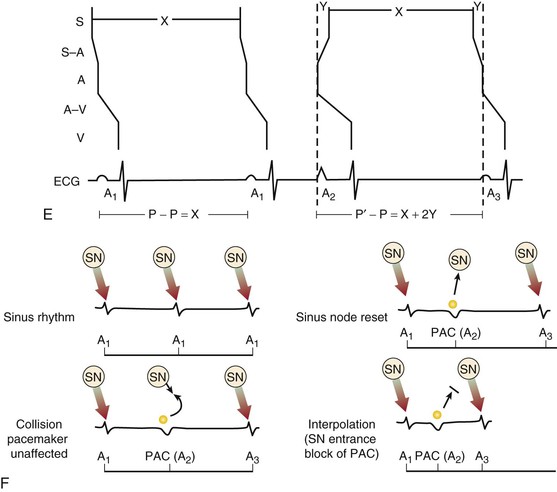
Jeffrey E. Olgin, Douglas P. Zipes Normal sinus rhythm is arbitrarily limited to impulse formation beginning in the sinus node at rates between 60 and 100 beats/minute. Infants and children generally have faster heart rates than adults do, both at rest and during exercise. The P wave is upright in electrocardiographic leads I, II, and aVF and negative in lead aVR, with a vector in the frontal plane of between 0 and +90 degrees. In the horizontal plane, the P vector is directed anteriorly and slightly leftward and can therefore be negative in leads V1 and V2 but positive in V3 to V6. The PR interval exceeds 120 milliseconds (msec) and can vary slightly with the rate. If the pacemaker site (site of impulse origin) shifts, a change in morphology of the P wave can occur. The rate of sinus rhythm varies significantly and depends on many factors, including age, sex, and physical activity. The sinus nodal discharge rate responds readily to autonomic stimuli. Steady vagal (parasympathetic) stimulation decreases the spontaneous sinus nodal discharge rate and predominates over steady sympathetic stimulation, which increases the spontaneous sinus nodal discharge rate. Rates lower than 60 beats/minute are considered to be bradycardia, and rates higher than 100 beats/minute are considered to be tachycardia. As described in Chapter 33, the normal sequence of electrical activation of the heart is from the sinus node through the atria to the atrioventricular (AV) node and His-Purkinje system and to the ventricular myocardium. Arrhythmias resulting in bradycardia or tachycardia can be thought of as specific disorders of each of these components. Specific tachyarrhythmias and bradyarrhythmias presented as disorders of this electrophysiologic (EP) hierarchy and their characteristics are summarized in Table 37-1. TABLE 37-1 Characteristics of Arrhythmias* * In an effort to summarize these arrhythmias in tabular form, generalizations have to be made. For example, the response to carotid sinus massage may be slightly different from what is listed. Acute therapy to terminate a tachycardia may be different from chronic therapy to prevent recurrence. Some of the exceptions are indicated in the footnotes; the reader is referred to text for a complete discussion. † P waves initiated by sinus node discharge may not be precisely regular because of sinus arrhythmia. ‡ Frequently, carotid sinus massage fails to slow a sinus tachycardia. § Any independent atrial arrhythmia may exit or the atria may be captured retrogradely. ‖ Constant if the atria are captured retrogradely. ¶ Atrial rhythm and rate may vary, depending on whether sinus bradycardia, sinus tachycardia, or another abnormality is the atrial mechanism. ** Regular or constant if block is unchanging. Modified from Zipes DP: Arrhythmias. In Andreoli K, Zipes DP, Wallace AG, et al (eds): Comprehensive Cardiac Care. 6th ed. St. Louis, CV Mosby, 1987. Tachyarrhythmias are broadly characterized as supraventricular tachycardia (SVT), defined as a tachycardia in which the driving circuit or focus originates, at least in part, in tissue above the level of the ventricle (i.e., sinus node, atria, AV node, or His bundle), and ventricular tachycardia (VT), defined as a tachycardia in which the driving circuit or focus originates solely in ventricular tissue or Purkinje fibers. Because of differences in prognosis and management, distinction between SVT and VT is critical early in the acute management of a tachyarrhythmia.1 In general (with the exception of idiopathic VT, described later), VT often carries a much graver prognosis, usually implies the presence of significant heart disease, results in more profound hemodynamic compromise, and therefore requires immediate attention and measures to revert to sinus rhythm. SVT is not usually lethal and often does not result in hemodynamic collapse; therefore, more conservative measures can be applied initially to convert to sinus rhythm.2,3 Distinction between SVT and VT can generally be made on the basis of the electrocardiogram (ECG) obtained during tachycardia (see Chapter 34).4 It is important to obtain a 12-lead ECG during tachycardia if possible and to obtain 12-lead (or at least multilead) rhythm strips during any intervention aimed at termination of the tachycardia because examining the termination (and initiation) can help identify the specific arrhythmia.5 In general, if the QRS is narrow (duration <120 msec, often referred to as narrow-complex tachycardias), the ventricle is being activated via the normal His-Purkinje system, and thus the origin of the tachycardia is supraventricular (Fig. 37-1). In contrast, a wide QRS (duration >120 msec) during tachycardia suggests VT; however, in some common scenarios SVT can produce a wide QRS complex. Therefore a more descriptive term, wide-complex tachycardia (WCT), is often used when the precise arrhythmia mechanism cannot be determined. For example, SVT with a concurrent bundle branch block or intraventricular conduction defect can produce WCTs despite a supraventricular origin. In addition, preexcited tachycardias (tachycardias in which the ventricle is activated in whole or in part over an accessory pathway) produce wide QRS complexes despite being supraventricular in origin. Therefore, although a narrow-complex tachycardia almost always makes the diagnosis of SVT, a WCT can be supraventricular or ventricular. Fusion or capture beats and AV dissociation are diagnostic of VT (discussed later, see Ventricular Tachycardia, Electrocardiographic Recognition) but are often not present or are difficult to detect. Criteria and algorithms have been developed to determine whether a WCT is more likely to be SVT or VT (see Differentiation between Ventricular and Supraventricular Tachycardia below).4 The general principles behind these algorithms rest on the assumption that the closer the QRS morphology is to a typical bundle branch block pattern, the more likely that it is an SVT and assumes that the septum is still rapidly activated in a WCT because of SVT. During sinus tachycardia (Fig. 37-2), the sinus node exhibits a discharge frequency between 100 and 180 beats/minute, but it can be higher with extreme exertion and in young individuals. The maximum heart rate achieved during strenuous physical activity varies widely but decreases with age. Sinus tachycardia generally has a gradual onset and termination. The P-P interval can vary slightly from cycle to cycle, especially at slower rates. P waves have a normal contour, a larger amplitude can develop, and the wave can become peaked. They appear before each QRS complex with a stable PR interval unless concomitant AV block ensues. Accelerated phase 4 diastolic depolarization of sinus nodal cells (see Chapter 33) is generally responsible for sinus tachycardia and is usually caused by elevated adrenergic tone or withdrawal of parasympathetic tone. Carotid sinus massage and Valsalva or other vagal maneuvers gradually slow sinus tachycardia, which then accelerates to its previous rate on cessation of the enhanced vagal tone. More rapid sinus rates can fail to slow in response to a vagal maneuver, particularly those driven by high adrenergic tone. Sinus tachycardia is common in infancy and early childhood and is the normal reaction to various physiologic or pathophysiologic stress, such as fever, hypotension, thyrotoxicosis, anemia, anxiety, exertion, hypovolemia, pulmonary emboli, myocardial ischemia, congestive heart failure, and shock. Drugs such as atropine, catecholamines, and thyroid medications, as well as alcohol, nicotine, caffeine, and amphetamines or other stimulants, can produce sinus tachycardia. Persistent sinus tachycardia can be a manifestation of heart failure. In patients with structural heart disease, sinus tachycardia can result in reduced cardiac output or angina or can precipitate another arrhythmia, in part related to the abbreviated ventricular filling time and compromised coronary blood flow. Sinus tachycardia can be a cause of inappropriate defibrillator discharge in patients with an implantable cardioverter-defibrillator (ICD; see Chapter 36). Chronic inappropriate sinus tachycardia (also known as the syndrome of inappropriate sinus tachycardia) has been described in otherwise healthy persons, possibly secondary to increased automaticity of the sinus node or an automatic atrial focus near the sinus node.6 The abnormality can result from a defect in either sympathetic or vagal nerve control of sinoatrial (SA) automaticity or from an abnormality of the intrinsic heart rate. In postural orthostatic tachycardia syndrome, a related syndrome consisting of orthostatic hypotension and sinus tachycardia, the cause of the orthostatic decrease in blood pressure is not hypovolemia or drugs. Both syndromes can result from autonomic neuropathy (either peripheral, as in diabetic patients, or central, from spinal cord injury). Sinus node reentry (Fig. e37-1 Management should focus on the cause of the sinus tachycardia. In the hospital inpatient setting, the cause is usually obvious (e.g., hemorrhage, sepsis, agitation), whereas in the outpatient setting, it may be more elusive. The most common reversible causes include hyperthyroidism, anemia, infection or inflammation, and hypovolemia. Diabetic neuropathy is also common but not reversible. Elimination of tobacco, alcohol, caffeine, or other stimulants, such as the sympathomimetic agents in nose drops and cold medications, may be helpful. Beta blockers and nondihydropyridine calcium channel blockers (verapamil and diltiazem), fluid replacement in a hypovolemic patient, or fever reduction in a febrile patient can help slow the sinus nodal discharge rate. Treatment of inappropriate sinus tachycardia requires beta blockers or calcium channel blockers, alone or in combination. In severe cases, sinus node radiofrequency (RF) or surgical ablation may be indicated; however, these approaches are usually only temporarily palliative (see Chapter 35). A specific blocker of the pacemaker current (If), ivabradine, has been useful in some patients with inappropriate or refractory sinus tachycardia. Premature complexes are among the most common causes of an irregular pulse and palpitations. They can originate from any area in the heart—most frequently from the ventricles, less often from the atria and the AV junctional area, and rarely from the sinus node. Premature complexes are common in normal hearts and increase in frequency with age. The diagnosis of premature atrial complexes (PACs) is made on the ECG (Fig. 37-3) by the presence of a premature P wave with a PR interval exceeding 120 milliseconds (except in Wolff-Parkinson-White [WPW] syndrome, in which case the PR interval is generally shorter than 120 msec). Although the contour of a premature P wave can resemble that of a normal sinus P wave, it generally differs. Even though variations in the basic sinus rate can at times make the diagnosis of prematurity difficult, differences in the contour of the P waves are usually apparent and indicate a different focus of origin. When a PAC occurs early in diastole, conduction may not be completely normal. The AV junction may still be refractory from the preceding beat and prevent propagation of the impulse (blocked or nonconducted PAC; Fig. 37-3A) or cause conduction to be slowed (PAC with a prolonged PR interval). As a general rule, the RP interval is inversely related to the PR interval; thus, a short RP interval produced by an early PAC occurring close to the preceding QRS complex is followed by a long PR interval. When PACs occur early in the cardiac cycle, the premature P waves can be difficult to discern because they are superimposed on T waves. Careful examination of tracings from several leads may be necessary before the PAC is recognized as a slight deformity of the T wave. Frequently, such PACs are blocked before reaching the ventricle and can be misinterpreted as a sinus pause or sinus exit block (see Fig. 37-3A). Less commonly, the PAC encounters a refractory sinus node or perinodal tissue (see Fig. 37-3F), in which case the timing of the basic sinus rhythm is not altered because the sinus node is not reset by the PAC and the interval between the two normal sinus-initiated P waves flanking the PAC is twice the normal P-P interval. The interval that follows this premature atrial discharge is said to be a full compensatory pause, that is, of sufficient duration that the P-P interval bounding the PAC is twice the normal P-P interval. However, sinus arrhythmia can lengthen or shorten this pause. Rarely, an interpolated PAC may occur. In this case the pause after the PAC is very short, and the interval bounded by the normal sinus-initiated P waves on each side of the PAC is equal to one normal P-P cycle length or slightly longer. The interpolated PAC fails to affect the sinus nodal pacemaker, and the sinus impulse that follows the PAC is conducted to the ventricles, often with a slightly lengthened PR interval. An interpolated atrial or ventricular premature complex of any type represents the only type of premature systole that does not actually replace the normally conducted beat. PACs can originate in the sinus node and are identified by premature P waves that have a contour identical to that of the normal sinus P wave. PACs can occur in various situations, such as during infection, inflammation, or myocardial ischemia, or they can be provoked by various medications, tension states, tobacco, alcohol, or caffeine. PACs can precipitate or presage the occurrence of sustained supraventricular (Fig. 37-3B, C) and, rarely, ventricular tachyarrhythmias. Frequently, PACs occur without any reversible causes and increase in frequency with aging. In general, PACs have a benign prognosis. Most patients do not have significant symptoms with PACs; however, those who do have symptoms most often feel the pauses that occur after the PAC. PACs generally do not require therapy. In symptomatic patients or when the PACs precipitate tachycardias, treatment with a beta blocker or a calcium antagonist can be attempted. In drug-refractory, highly symptomatic cases, ablation of the PAC focus can be effective when a single focus can be identified. Three types of atrial tachycardia have been distinguished experimentally—automatic, triggered, and reentrant. Entrainment, resetting patterns in response to overdrive pacing, the patient’s response to adenosine, recording of monophasic action potentials, and the mode of initiation may suggest the presence of one of these mechanisms. However, in most cases no clear identification of the mechanism can be made clinically because the clinical and EP features can overlap, especially when the reentrant circuit is small (i.e., microreentry). For example, adrenergic stimulation can initiate automatic and triggered atrial tachycardias, and burst pacing may initiate triggered and microreentrant atrial tachycardias. Therefore, because it determines the approach to mapping and management, atrial tachycardias are more broadly characterized clinically as being focal (originating from a small area of the atrium with atrial excitation emanating from this focus) or macroreentrant (a relatively large reentrant circuit using conduction barriers to create the circuit).7 Atrial flutter is the most common type of macroreentrant atrial tachycardia. Atrial flutter is the prototypic macroreentrant atrial rhythm. The typical atrial flutter is a reentrant rhythm in the right atrium that is constrained anteriorly by the tricuspid annulus and posteriorly by the crista terminalis and eustachian ridge. The flutter can circulate in a counterclockwise direction around the tricuspid annulus in the frontal plane (typical flutter, counterclockwise flutter) or in a clockwise direction (atypical, clockwise, or reverse flutter). Because both these forms of atrial flutter use the same circuit and are constrained by the same anatomic structures, their rates and flutter wave morphology on the surface ECG are consistent and predictable (see later). Rarely, intra-isthmus flutter can occur when the reentrant circuit is isolated to the cavotricuspid isthmus rather than rotating around the entire tricuspid annulus; this typically occurs after ablation in this region (usually done as treatment of typical flutter). Other forms of atrial flutter are now recognized as distinct types and include atrial macroreentry caused by incisional scars from previous atrial surgery, previous atrial ablation, mitral annular flutter, idiopathic fibrosis in areas of the atrium, or other anatomic or functional barriers to conduction in the atria. Because the barriers that constrain these atrial flutters are variable, the electrocardiographic pattern of these so-called atypical atrial flutters can be varied. Sometimes, flutter wave morphology changes during the same episode of flutter, which indicates multiple circuits or nonfixed conduction barriers. The atrial rate during typical atrial flutter is usually 250 to 350 beats/minute, although it is occasionally slower, particularly when the patient is treated with antiarrhythmic drugs, which can reduce the rate to about 200 beats/minute. If such slowing occurs, the ventricles can respond in a 1:1 fashion to the slower atrial rate. In typical atrial flutter, the ECG reveals identically recurring, regular, sawtooth flutter waves (see Fig. 37-3C) and evidence of continual electrical activity (lack of an isoelectric interval between flutter waves), often best visualized in leads II, III, aVF, or V1 (Fig. 37-4).8 In some cases, transient slowing of the ventricular response, via either carotid sinus massage or adenosine, is necessary to visualize the flutter waves. The flutter waves for the most common form of atrial flutter, counterclockwise typical atrial flutter, are inverted (negative) in these leads because of a counterclockwise reentrant pathway, and sometimes they are upright (positive) when the reentrant loop is clockwise (see Fig. 37-4). When the flutter waves are upright from clockwise rotation, they are often notched. If the AV conduction ratio remains constant, the ventricular rhythm will be regular; if the ratio of conducted beats varies (generally the result of a Wenckebach AV block), the ventricular rhythm will be irregular, although this is rare. Various degrees of penetration into the AV junction by flutter impulses can also influence AV conduction. The ratio of flutter waves to conducted ventricular complexes is most often an even number (e.g., 2:1, 4:1). As mentioned earlier, because the circuits for atypical flutter (not involving the cavotricuspid isthmus) can be variable, the electrocardiographic features of these macroreentrant atrial tachycardias are highly variable, without consistent rates or flutter wave contours (see Fig. e37-2 Atrial flutter is less common than atrial fibrillation. It can occur as a result of atrial dilation from septal defects, pulmonary emboli, mitral or tricuspid valve stenosis or regurgitation, heart failure, previous extensive atrial ablation, and aging, but it can also occur without underlying heart disease. Toxic and metabolic conditions that affect the heart, such as thyrotoxicosis, alcoholism, and pericarditis, can cause atrial flutter. It can follow surgical repair of congenital heart disease. When it follows reparative surgery for congenital heart disease, most patients will be able to have both typical flutter and atypical flutter involving the atriotomy, which often occurs years after the surgery. Atrial flutter usually responds to carotid sinus massage with a decrease in the ventricular rate in stepwise multiples and returns in reverse manner to the former ventricular rate at the termination of carotid massage. Physical examination may reveal rapid flutter waves in the jugular venous pulse. If the relationship of flutter waves to conducted QRS complexes remains constant, the first heart sound will have a constant intensity. Sounds caused by atrial contraction can occasionally be auscultated. Cardioversion (see Chapter 35) is commonly the initial treatment of choice for atrial flutter because it promptly and effectively restores sinus rhythm. Cardioversion can be accomplished with synchronous direct current (DC), which often requires relatively low energy (≈50 J). If the electrical shock results in atrial fibrillation, a second shock at a higher energy level is used to restore sinus rhythm, or depending on clinical circumstances, the atrial fibrillation can be left untreated and can revert to atrial flutter or sinus rhythm. The short-acting antiarrhythmic medication ibutilide can also be given intravenously to convert atrial flutter. Ibutilide appears to successfully cardiovert approximately 60% to 90% of episodes of atrial flutter. However, because this medication prolongs the QT interval, torsades de pointes is a potential complication during and shortly after the infusion. Other medications, such as procainamide or amiodarone, can be given to convert atrial flutter chemically, but they are generally less effective than ibutilide. Rapid atrial pacing with a catheter in the esophagus or the right atrium can effectively terminate typical and some forms of atypical atrial flutter in most patients. Because ablation is highly effective for typical flutter and because of the high relapse rate after cardioversion, ablation is the preferred approach for stable patients who do not require immediate cardioversion. Although the risk for thromboembolism is lower than that for atrial fibrillation, patients with atrial flutter do appear to have a risk for thromboembolism immediately after conversion to sinus rhythm. In general, indications for anticoagulation in patients with atrial flutter are similar to those in patients with atrial fibrillation.
Specific Arrhythmias
Diagnosis and Treatment
Normal Sinus Rhythm
TYPE OF ARRHYTHMIA
P WAVES
QRS COMPLEXES
VENTRICULAR RESPONSE TO CAROTID SINUS MASSAGE
PHYSICAL EXAMINATION
TREATMENT
Rate (Beats/min)
Rhythm
Contour
Rate (Beats/min)
Rhythm
Contour
Intensity of S1
Splitting of S2
A Waves
Sinus rhythm
60-100
Regular†
Normal
60-100
Regular
Normal
Gradual slowing and return to former rate
Constant
Normal
Normal
None
Sinus bradycardia
<60
Regular
Normal
<60
Regular
Normal
Gradual slowing and return to former rate
Constant
Normal
Normal
None, unless symptomatic; atropine
Sinus tachycardia
100-180
Regular
May be peaked
100-180
Regular
Normal
Gradual slowing‡ and return to former rate
Constant
Normal
Normal
None, unless symptomatic; treat underlying disease
AV nodal reentry
150-250
Very regular except at onset and termination
Retrograde; difficult to see; lost in QRS complex
150-250
Very regular except at onset and termination
Normal
Abrupt slowing caused by termination of tachycardia or no effect
Constant
Normal
Constant cannon a waves
Vagal stimulation, adenosine, verapamil, digitalis, propranolol, DC shock, pacing
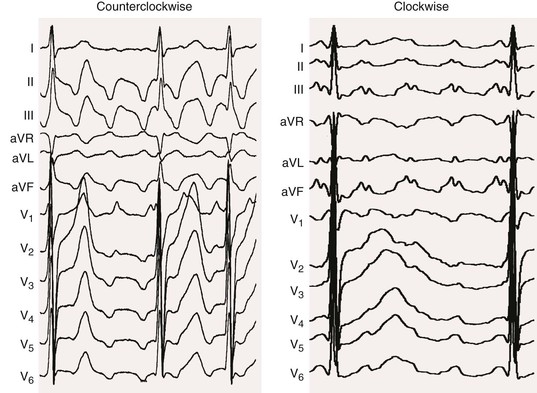
Atrial flutter
250-350
Regular
Sawtooth
75-175
Generally regular in absence of drugs or disease
Normal
Abrupt slowing and return to former rate; flutter remains
Constant; variable if AV block changing
Normal
Flutter waves
DC shock, digitalis, quinidine, propranolol, verapamil, adenosine
Atrial fibrillation
400-600
Grossly irregular
Baseline undulation, no P waves
100-160
Grossly irregular
Normal
Slowing; gross irregularity remains
Variable
Normal
No a waves
Digitalis, quinidine, DC shock, verapamil, adenosine
Atrial tachycardia with block
150-250
Regular; may be irregular
Abnormal
75-200
Generally regular in absence of drugs or disease
Normal
Abrupt slowing and return to normal rate; tachycardia remains
Constant; variable if AV block changing
Normal
More a waves than c-v waves
Stop digitalis if toxic; digitalis if not toxic; possibly verapamil
AV junctional rhythm
40-100§
Regular
Normal
40-60
Fairly regular
Normal
None; may be slight slowing
Variable‖
Normal
Intermittent cannon waves
None, unless symptomatic; atropine
Reciprocating tachycardias using an accessory (WPW) pathway
150-250
Very regular except at onset and termination
Retrograde; difficult to see; monitor the QRS complex
150-250
Very regular except at onset and termination
Normal
Abrupt slowing caused by termination of tachycardia or no effect
Constant but decreased
Normal
Constant cannon waves
See AV nodal reentry earlier
Nonparoxysmal AV junctional tachycardia
60-100‖
Regular
Normal
70-130
Fairly regular
Normal
None; may be slight slowing
Variable‖
Normal
Intermittent cannon waves‖
None, unless symptomatic; stop digitalis if toxic
Ventricular tachycardia
60-100‖
Regular
Normal
110-250
Fairly regular; may be irregular
Abnormal, >0.12 sec
None
Variable‖
Abnormal
Intermittent cannon waves‖
Lidocaine, procainamide, DC shock, quinidine, amiodarone
Accelerated idioventricular rhythm
60-100‖
Regular
Normal
50-110
Fairly regular; may be irregular
Abnormal, >0.12 sec
None
Variable‖
Abnormal
Intermittent cannon waves‖
None, unless symptomatic; lidocaine, atropine
Ventricular flutter
60-100‖
Regular
Normal; difficult to see
150-300
Regular
Sine wave
None
Soft or absent
Soft or absent
Cannon waves
DC shock
Ventricular fibrillation
60-100‖
Regular
Normal; difficult to see
400-600
Grossly irregular
Baseline undulations; no QRS
None
None
None
Cannon waves
DC shock
First-degree AV block
60-100¶
Regular
Normal
60-100
Regular
Normal
Gradual slowing caused by sinus
Constant, diminished
Normal
Normal
None
Type I second-degree AV block
60-100¶
Regular
Normal
30-100
Irregular**
Normal
Slowing caused by sinus slowing and an increase in AV block
Cyclical decrease, then increase after pause
Normal
Normal; increasing a-c interval; a waves without c waves
None, unless symptomatic; atropine
Type II second-degree AV block
60-100¶
Regular
Normal
30-100
Irregular¶
Abnormal, >0.12 sec
Gradual slowing caused by sinus slowing
Constant
Abnormal
Normal; constant a-c interval; a waves
Pacemaker
Complete AV block
60-100‖
Regular
Normal
<40
Fairly regular
Abnormal, 0.12 sec
None
Variable¶
Abnormal
Intermittent cannon waves¶
Pacemaker
Right bundle branch block
60-100
Regular
Normal
60-100
Regular
Abnormal, 0.12 sec
Gradual slowing and return to former rate
Constant
Wide
Normal
None
Left bundle branch block
60-100
Regular
Normal
60-100
Regular
Abnormal, >0.12 sec
Gradual slowing and return to former rate
Constant
Paradoxical
Normal
None
Tachyarrhythmias
Supraventricular Rhythm Disturbances
Sinus Tachycardia
Electrocardiographic Recognition
Clinical Features
![]() ) is an atrial tachycardia originating from tissue near the sinus node and thus has a P wave morphology similar to sinus rhythm (see the section Focal Atrial Tachycardias).
) is an atrial tachycardia originating from tissue near the sinus node and thus has a P wave morphology similar to sinus rhythm (see the section Focal Atrial Tachycardias).
Management
Premature Atrial Complexes
Electrocardiographic Recognition
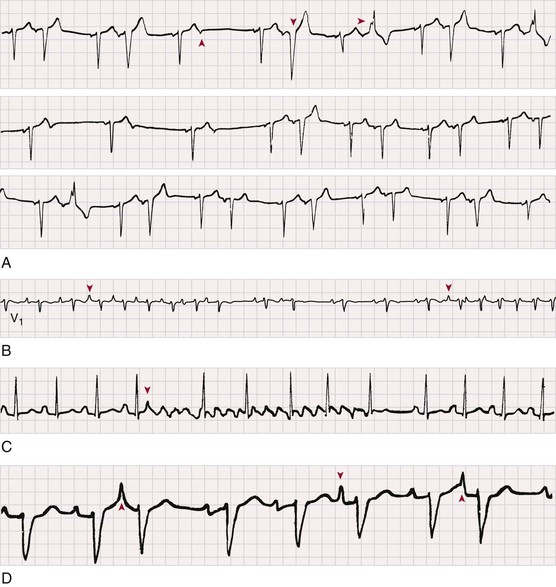
Clinical Features
Management
Atrial Tachycardias
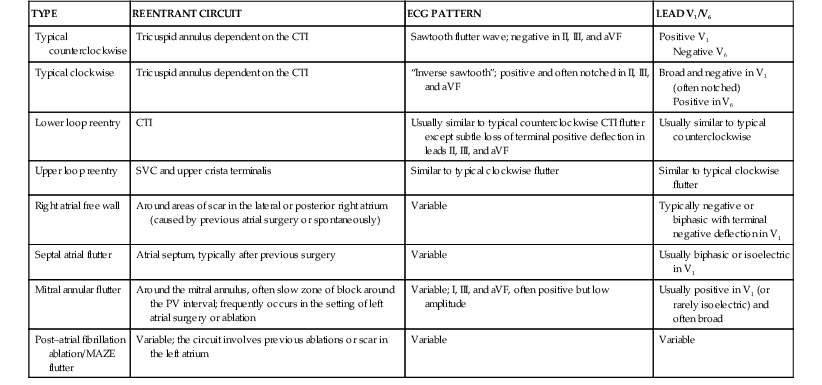
Atrial Flutter and Other Macroreentrant Atrial Tachycardias
Electrocardiographic Recognition
![]() ). However, these tachycardias frequently have a flutter rate similar to that of typical flutter (250 to 390 beats/min). Table 37-2 shows common electrocardiographic findings with the different types of macroreentrant atrial flutter. After extensive left atrial ablation for atrial fibrillation, the electrocardiographic pattern of even typical flutter can appear “atypical” (not have the typical appearance described before) because of the altered left atrial activation as a result of altered conduction secondary to the left atrial ablation. In addition, unusual forms of atrial flutter can occur around ablation lines.
). However, these tachycardias frequently have a flutter rate similar to that of typical flutter (250 to 390 beats/min). Table 37-2 shows common electrocardiographic findings with the different types of macroreentrant atrial flutter. After extensive left atrial ablation for atrial fibrillation, the electrocardiographic pattern of even typical flutter can appear “atypical” (not have the typical appearance described before) because of the altered left atrial activation as a result of altered conduction secondary to the left atrial ablation. In addition, unusual forms of atrial flutter can occur around ablation lines.
Clinical Features
Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree