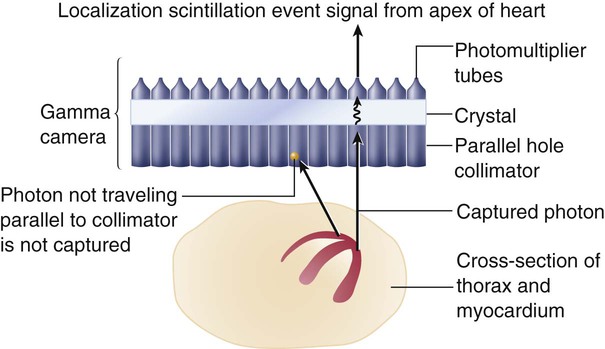
The medical workup of a patient with cardiac or pulmonary disease uses many tools. The patient interview, physical examination, chest x-ray, and electrocardiogram (ECG) can provide adequate information to make a diagnosis. However, when the diagnosis remains unclear, special tests that use more complex technology are required. These special tests may be invasive or noninvasive. Current clinical practice guidelines recommend that noninvasive tests be performed before invasive tests such as angiography.1 Nuclear medicine offers a variety of noninvasive tests for evaluation of cardiac and pulmonary function. Echocardiography, which is particularly useful in children, is another noninvasive method that provides information about the cardiovascular system, including valve function, ventricular performance, and estimation of filling pressures. In comparison with cardiovascular tests, special tests of the respiratory system are less commonly required in the initial diagnosis and treatment of disease. The generally high-quality information obtained from standard x-ray examination in combination with the patient’s respiratory symptoms, pulmonary function tests, and the results of the physical examination is usually sufficient, making special tests unnecessary.2–4 Various factors are involved in the decision of which special tests may be beneficial for a patient. These tests afford different benefits to patients in terms of diagnostic and prognostic accuracy (Table 14-1). Radiation exposure is an additional factor in selection of tests. These tests have helped to clarify our understanding of the physiology and pathophysiology of the cardiovascular and pulmonary system. Therapists need to understand the pathophysiological basis for a patient’s movement dysfunctions to select the most appropriate treatment strategies. Many special tests can and are being used in research to evaluate treatment interventions. For example, radiolabeled aerosols are used to evaluate mucociliary clearance, providing a method to evaluate the effectiveness of pulmonary hygiene techniques.5 Clinically, physical therapists need to use information from the tests to develop a framework for predicting how the patient may respond to a physical therapy intervention. For example, nuclear imaging can provide information about left ventricular ejection fraction. This information helps the therapist determine whether the patient should be stratified into high-risk or low-risk categories. Monitoring of an exercise session may depend on the risk level, and the therapist’s interpretation of the patient’s response to treatment may be affected by this information. Table 14-1 Tests Used for Diagnosis and Prognosis in Ischemic Heart Disease *Data vary according to studies included and characteristics of patient group studied (e.g., number of coronary vessels involved). From Kones R: Recent advances in the management of chronic stable angina. I: Approach to the patient, diagnosis, pathophysiology, risk stratification, and gender disparities. Vascular Health and Risk Management 6:635-656, 2010. Radionuclide imaging allows for the noninvasive acquisition of images from a variety of body tissues. An imaging system requires three basic parts. The first requirement is a radiopharmaceutical that emits gamma radiation and is taken up by the body tissue of interest. Next, a radiation detector or camera is needed. Finally, computers are required to collect and analyze the data. Thus an image is formed based on the brightness of the tissue, which is proportional to the radiation the tissue has absorbed.6 Radionuclides are elements that are unstable; they gain stability by emitting particles or photons. This is called radioactive decay, and gamma radiation is released. Radionuclides are either cyclotron- or generator-produced. The cyclotron accelerates alpha-particles, deuterons, and protons to energies suitable for the production of the required radionuclide.7 A radionuclide generator is a system of a long-lived parent radionuclide which decays to produce a daughter radionuclide. The system is constructed so that the daughter can be removed (called “milking” the generator) for patient use.8 A generator system produces short-lived radionuclides.7 Detection of the radioactive energy emitted by a specific radiopharmaceutical requires a camera. When certain materials are struck by ionizing radiation, light is emitted. A scintillation detector detects this light (Figure 14-1). A gamma camera (a scintillation detector) is able to detect photons exiting the body. It uses a large, collimated crystal monitored by an array of photomultiplier tubes.9 A collimator is a device that allows only those photons traveling in an appropriate direction to reach the crystal. There are several types of collimators: parallel-hole collimators (most common), pinhole collimators, and converging and diverging collimators. The photomultiplier tube records the amount of light from the crystal and converts it into a voltage that is proportional to the intensity of the light.9 The camera system is connected to a computer that stores the light images. The computer is able to derive two-dimensional images from the data. The newer software algorithms that construct the images have improved, allowing for better spatial resolution, contrast, and overall image quality.10 A single crystal camera produces a two dimensional or planar image. Multicrystal cameras also produce planar images but are also able to perform fast, dynamic imaging used in first pass and gated studies. Planar images are unable to reflect the depth of an image.6 Planar imaging may be the only option available for obese patients who do not “fit” the single-photon emission computed tomography (SPECT) camera or for those who cannot remain immobile.11 The most common nuclear study of myocardial perfusion is SPECT imaging.12 Planar images or tomographs are collected by rotating the scintillation detectors (single-, dual-, or triple-head scintillation cameras) around the patient, taking images at 3-degree increments.10 SPECT images allow for quantification of radioactivity.6 However, attenuation artifacts (absorption of the radioactive energy by other body tissues, preventing the camera from detecting it) are greater than with planar images, particularly those images involving the inferior wall of the heart in men and the anterior wall in women, and may produce false-positive scans.13 Quality of the images is dependent on the use of stringent quality control measures and experience of the staff. The most commonly used radionuclides are thallium-201, technetium-99m-sestamibi, and technetium-99m-tetrofosmin.14 Image quality is also patient-dependent. Patients must be able to lie perfectly still for 15 to 20 minutes, with their hands over their heads, while data are collected. Newer systems allow for a 50% reduction in duration of the scan as well as reduced processing time.10 Currently available systems also allow for seated positioning.10 Positron emission tomography (PET) uses positron-emitting radionuclides. The images obtained by PET can provide information regarding cellular processes in living tissue. Both cardiac and lung tissue can be quantitatively examined to obtain information on metabolism, blood perfusion, tissue viability, autonomic regulation, and other processes.15,16 PET is valuable in delineating myocardial areas with reversible and irreversible injury, thus assessing the feasibility of revascularization, in patients with CAD or left ventricular dysfunction.17 PET studies use short scanning times (5-35 minutes) and because of the short half-life of PET tracers, multiple scans can be repeated in a relatively short period of time.18 Although PET scans offer superior imaging, in part due to greater correction for attenuation artifact,18 PET’s technological complexity, short-lived radio tracers, and specialized equipment have resulted in high costs and more limited clinical application.15 However, because of increased PET use in oncology imaging, opportunities for imaging of the cardiorespiratory system are now increasing.15,19 Current FDA-approved PET nuclides are rubidium-82 (82-Rb), 13N-ammonia (13-NH3), and fluorine-18-fluorodeoxyglucose (18F-FDG).15 In a first-pass study, data are collected on a radiolabeled bolus of blood as it passes through the cardiac chambers, which is one method of radionuclide angiography. A first-pass scan allows for clear identification of the four cardiac chambers. During first-pass studies, data are collected over several cardiac cycles. Gated equilibrium studies or multiple-uptake gated acquisition (MUGA) scans average several hundred cardiac cycles. Each R wave of the ECG triggers the acquisition of data; thus the average cycle observed is the compilation of many cycles.20 The quality of the image is best when the patient has a stable sinus rhythm. Patients with irregular heart rates, such as atrial fibrillation, have images of poorer quality. Many of the tests performed using radionuclides are coupled with an exercise test. A treadmill exercise test is performed, and at the peak of exercise, a tracer is injected. Shortly afterward, depending on the tracer, images are acquired. Exercise imaging enhances the identification of ischemic areas. These tests are then often compared with rest studies. Rest/stress or stress/rest protocols can be used.14 The most important of these measurements, the ejection fraction (right and left ventricle) is a measure of myocardial function. It is derived from quantitative counts of the ventricular area during diastole and systole:20 Left ventricular ejection fraction (LVEF) has been shown to be predictive of mortality. LVEF normally ranges from 50% to 85%, whereas LVEF below 40% indicates moderate-severe congestive heart failure.21,22 LVEF is linearly related to mortality up to 45% EF23 and inversely related to moderate- to large-sized infarcts.24 Right ventricular ejection fraction (RVEF) has been found to have a wide range of normal values (35% to 75%)25 but is not as predictive of function without data about wall motion abnormalities. The strongest radionuclide predictor of outcome is the exercise LVEF. In terms of predicting outcome, multivariate analysis of clinical data, catheterization data, and radionuclide measurements have shown that the radionuclide results (exercise LVEF, resting end-diastolic volume, and change in heart rate [HR]) have the same prognostic power as the catheterization data.26 Another important measurement is wall motion. Quantitative and qualitative evaluation of the movement of the myocardium can be made. Wall thickness and wall movement are compared during systole and diastole. Assessments are made about akinesia (absence of wall motion), global or regional hypokinesia (reduction of wall motion), and dyskinesia (outward bulging of the wall during systole).27 Global left ventricular function is a strong predictor of survival. It can help clinicians differentiate a weak heart from one that is stiff, thus allowing the appropriate treatment. Regional wall function, when correlated with knowledge of coronary artery anatomy, allows for the identification of potential blockages or areas of infarct. Assessing wall thickness can provide information about hypertrophy or aneurysm but is done more reliably by echocardiography. Infarct size is an important measurement because it predicts short-term mortality. It is also important as a clinical endpoint in determining the effectiveness of reperfusion strategies and pharmacological interventions, which should result in a decrease of infarct size.28 The size of a myocardial infarct can be estimated by using the measurements previously described (i.e., ejection fraction, end-systolic volume, and global or regional wall motion).28 Perfusion defects of the myocardium can be measured acutely, after intervention, or during recovery. Anatomical measurements of chamber size can be made. From these data, chamber volume can be calculated, as well as stroke volume and cardiac output (Table 14-2). Table 14-2 Summary of Special Tests of the Cardiopulmonary System Perfusion of the myocardium is a vital factor in the viability and function of the heart. Information about myocardial perfusion is used for diagnostic decisions, treatment decisions, and prognosis. Perfusion of the myocardium under rest and exercise conditions is important in the diagnosis of CAD. For those patients with left ventricular dysfunction, identifying tissue that is viable but still at risk is critical for improving long-term outcomes. The efficacy of reperfusion strategies, such as angioplasty and thrombolytic therapy, must be evaluated. Information gained from combined perfusion and metabolic studies has helped to increase the understanding of ischemic myocardium. Two types of contractile dysfunction have been delineated. Hibernating myocardium is the result of prolonged ischemia. In this case, the contractility of the muscle fiber is affected so that there may appear to be regional wall motion abnormalities. The tissue is alive but not contracting. It is theorized that this is a measure to reduce energy expenditure and ensure myocyte survival. The second condition, myocardial stunning, occurs under conditions of acute ischemia. In this case, contractile dysfunction that initially occurs during the acute ischemic episode persists for some time after perfusion has returned to normal. Both conditions are reversible. Patients demonstrating hibernating myocardium may benefit from revascularization procedures. Patients with stunned myocardium may only require supportive care until contractile function returns.29 The type of imaging performed and the specific radiopharmaceutical used provides similar but not necessarily interchangeable imaging results and measurements.14 Thallium-201 (201-TL) is the oldest radioactive isotope used in myocardial perfusion studies.14 It is a cyclotron-produced isotope that emits low-energy radiation (68 to 80 kiloelectron volts [keV]). Thallium concentrations in the myocardium depend on 201-TL properties, uptake, and redistribution. Administered intravenously, its distribution throughout the body depends on blood flow, and within 5 minutes, myocardial uptake represents myocardial blood flow.30 The first-pass myocardial uptake coefficient is 85%.14 Extraction from the blood and transport across the cell membrane depend on active and passive transport mechanisms. Thallium is transported across the cell membrane through the sodium-potassium ATPase pump and facilitative diffusion.30 Normally perfused and functioning myocardium will take up the thallium tracer, while damaged cells will demonstrate weaker uptake. Within 15 minutes thallium begins washout (also called redistribution) in normal myocardium. Images taken 4-24 hours later show clearance of the 201-Tl, again as a result of normal blood flow, while abnormal cells have more tracer as it has not been washed out.30 Thallium-201 images can be qualitatively and quantitatively evaluated. Normally perfused myocardium demonstrates uniform uptake of the tracer. Uniform uptake can occur as long as blockages are less than 50% of the artery. Ischemic but viable myocardium initially appears as areas of decreased uptake; these areas fill in over time, a function of redistribution. Because blood flow is decreased, clearance of thallium-201 from the defect region is slower.30 In infarcted areas, these defects remain unchanged over time. Thus perfusion and tissue viability can be assessed with 201-TL. Qualitative evaluation requires visual inspection of the images. Quantitative evaluation is performed by specialized software individual to the imaging system used. The computer analyzes the amount of radioactivity taken up in a particular region of interest (ROI). It then quantifies this count so that regions can be compared with each other and with normalized data. In this way, unperfused or hypoperfused areas can be identified (Figure 14-2).
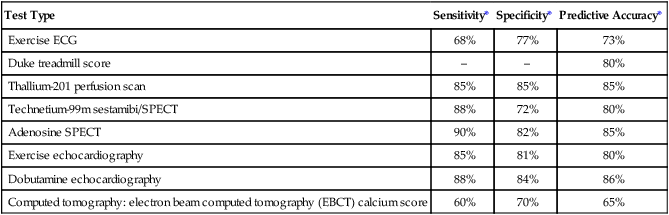
Special Tests
Test Type
Sensitivity*
Specificity*
Predictive Accuracy*
Exercise ECG
68%
77%
73%
Duke treadmill score
–
–
80%
Thallium-201 perfusion scan
85%
85%
85%
Technetium-99m sestamibi/SPECT
88%
72%
80%
Adenosine SPECT
90%
82%
85%
Exercise echocardiography
85%
81%
80%
Dobutamine echocardiography
88%
84%
86%
Computed tomography: electron beam computed tomography (EBCT) calcium score
60%
70%
65%
Nuclear Imaging Systems
Physics of Nuclear Imaging
Planar Imaging
Single-Photon Emission Computed Tomography Imaging
Positron Emission Tomography
Nuclear Tests of the Cardiovascular System
First-Pass and Gated Equilibrium Scans
Exercise Stress Studies
Nuclear-Derived Measurements
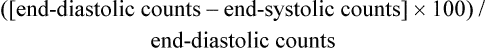
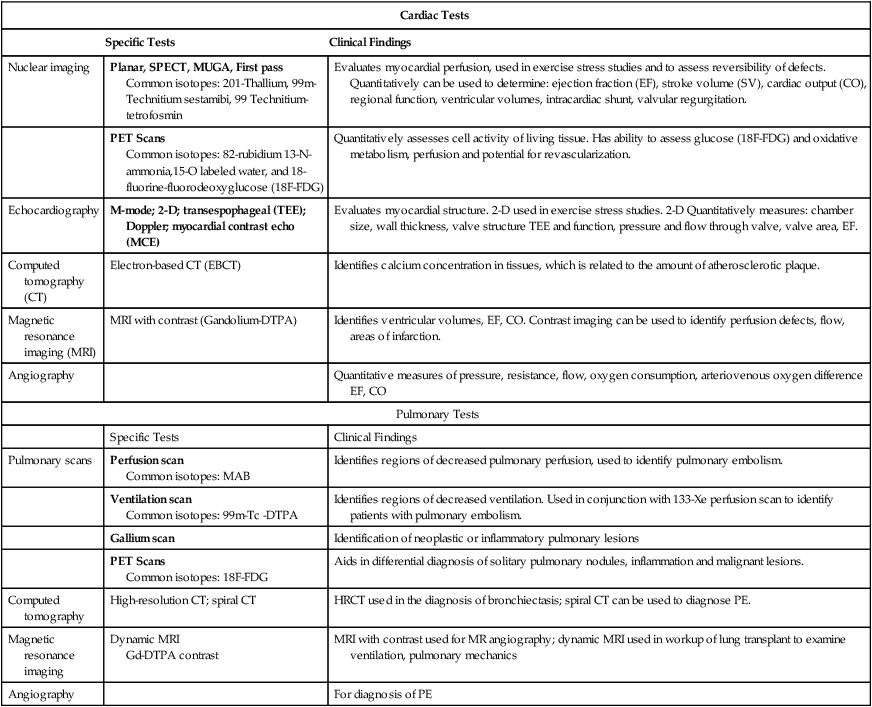
Cardiac Tests
Specific Tests
Clinical Findings
Nuclear imaging
Planar, SPECT, MUGA, First pass
Common isotopes: 201-Thallium, 99m-Technitium sestamibi, 99 Technitium-tetrofosmin
Evaluates myocardial perfusion, used in exercise stress studies and to assess reversibility of defects. Quantitatively can be used to determine: ejection fraction (EF), stroke volume (SV), cardiac output (CO), regional function, ventricular volumes, intracardiac shunt, valvular regurgitation.
PET Scans
Common isotopes: 82-rubidium 13-N-ammonia,15-O labeled water, and 18-fluorine-fluorodeoxyglucose (18F-FDG)
Quantitatively assesses cell activity of living tissue. Has ability to assess glucose (18F-FDG) and oxidative metabolism, perfusion and potential for revascularization.
Echocardiography
M-mode; 2-D; transespophageal (TEE); Doppler; myocardial contrast echo (MCE)
Evaluates myocardial structure. 2-D used in exercise stress studies. 2-D Quantitatively measures: chamber size, wall thickness, valve structure TEE and function, pressure and flow through valve, valve area, EF.
Computed tomography (CT)
Electron-based CT (EBCT)
Identifies calcium concentration in tissues, which is related to the amount of atherosclerotic plaque.
Magnetic resonance imaging (MRI)
MRI with contrast (Gandolium-DTPA)
Identifies ventricular volumes, EF, CO. Contrast imaging can be used to identify perfusion defects, flow, areas of infarction.
Angiography
Quantitative measures of pressure, resistance, flow, oxygen consumption, arteriovenous oxygen difference EF, CO
Pulmonary Tests
Specific Tests
Clinical Findings
Pulmonary scans
Perfusion scan
Common isotopes: MAB
Identifies regions of decreased pulmonary perfusion, used to identify pulmonary embolism.
Ventilation scan
Common isotopes: 99m-Tc -DTPA
Identifies regions of decreased ventilation. Used in conjunction with 133-Xe perfusion scan to identify patients with pulmonary embolism.
Gallium scan
Identification of neoplastic or inflammatory pulmonary lesions
PET Scans
Common isotopes: 18F-FDG
Aids in differential diagnosis of solitary pulmonary nodules, inflammation and malignant lesions.
Computed tomography
High-resolution CT; spiral CT
HRCT used in the diagnosis of bronchiectasis; spiral CT can be used to diagnose PE.
Magnetic resonance imaging
Dynamic MRI
Gd-DTPA contrast
MRI with contrast used for MR angiography; dynamic MRI used in workup of lung transplant to examine ventilation, pulmonary mechanics
Angiography
For diagnosis of PE
Myocardial Perfusion Imaging
Thallium-201
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Special Tests