Special Considerations in Complex Infrarenal Aortic Aneurysms
Margaret L. Schwarze
Benjamin J. Pearce
Bruce L. Gewertz
This chapter reviews the diagnostic and surgical approaches to a wide range of aortic pathology and anomalies that may be encountered during aortic aneurysm repair. We comment on the importance of thorough pre-operative evaluation, which is appropriately centered on obtaining all necessary anatomic information using newer imaging techniques, such as computed tomography (CT) angiography and magnetic resonance imaging (MRI). Our discussions are then focused on the judgments that frequently need to be made in the operating room to secure adequate exposure and safe aneurysm repair.
Inflammatory Aneurysms
About 5% of all abdominal aortic aneurysms (AAA) present with the classic findings of an inflammatory aneurysm: thickened aneurysm wall, extensive peri-aortic and retroperitoneal fibrosis, and dense adhesions to adjacent organs. The duodenum is involved in 90% of inflammatory aneurysms, while the surrounding venous structures and ureters are involved in one half and one quarter of patients, respectively. The degree and extent of retroperitoneal fibrosis are quite variable and, for yet unknown reasons, will often regress after exclusion of the aneurysm.
Recent research into the pathogenesis of arterial aneurysmal disease has demonstrated a role for inflammation in the formation of virtually all types of aneurysms, leading some to suggest that inflammatory aortic aneurysms (IAA) are not a distinct entity but simply a subgroup of atherosclerotic aneurysms with more prominent inflammatory components. It is hypothesized that enlargement of the aorta occasionally leads to occlusion of lymphatic channels in the vessel wall with stasis of immunemodulating cells and exacerbation of the inflammatory response. This theory is supported by the predominance of the fibrotic reaction in the anterolateral aspects of the IAA where the lymphatic network is more dense than the posterior aorta. The adventitia of the aorta in IAA is infiltrated with T lymphocytes, plasma cells, and macrophages to a greater extent than “bland” atherosclerotic aneurysms. IAA are also distinct in the amount of edema within the aortic wall, with enlarged medial and adventitial layers, which often exceed 2 cm in thickness.
Diagnosis and Pre-operative Assessment
In addition to the presence of a pulsatile abdominal mass, many patients with IAA have a history of back or abdominal pain related to the aneurysm. Weight loss, resulting from both abdominal pain and, less commonly, partial small bowel obstruction, is seen in approximately 20% of cases. Although smoking is a common risk factor in most patients with aneurysms, patients with IAA have nearly a 100% incidence of tobacco use. A patient presenting with IAA is typically 5 to 10 years younger than a patient with “bland” AAA and often has a larger aneurysm at time of diagnosis. About 25% of patients have tenderness to palpation.
Laboratory testing is useful but not specific in the diagnosis of IAA. Erythrocyte sedimentation rate (ESR) is elevated in many patients but is not diagnostic or specific. C-reactive protein is higher in patients with IAA than those with noninflammatory lesions, although it is unclear whether this relates to the larger size of the aneurysms at time of diagnosis. Because of the potential for encasement of the ureters by retroperitoneal fibrosis, the incidence of renal impairment from obstructive uropathy is as high as 15% in some series.
In the years since Walker’s original description in 1972, the increased availability and detail offered by CT has improved evaluation of aneurysmal disease and, hence, pre-operative recognition of IAA. Contrast CT will diagnose an inflammatory aneurysm with 90% sensitivity. The characteristic sign is a thickened aortic wall (“inflammatory rind”) that enhances with infusion of intravenous contrast. Presence of an inner calcific ring surrounded by thickened media and adventitia with posterior wall sparing are additional radiographic findings associated with IAA. CT can also accurately demonstrate the extent of retroperitoneal fibrosis and the presence of ureteral obstruction and hydronephrosis. Virtually all important morphologic information, including aneurysm size, presence of thrombus, iliac artery involvement, visceral artery patency, and suitability for endograft placement, can be determined by CT.
For those patients in whom intravenous contrast agents are contraindicated, MRI and MR angiography (MRA) have been shown to be effective in diagnosis and evaluation. Tl-weighted images displaying alternating areas of high and low signal intensity in the aortic wall are considered diagnostic of IAA. Like CT, MR also has the advantage of demonstrating associated abdominal and retroperitoneal pathology.
It is important to note that other arterial lesions may have similar characteristics. An infected or mycotic aneurysm often presents with abdominal pain, elevations in ESR, and an asymmetric, enhancing mass around the aneurysm. Infected aneurysms can be distinguished by the presence of other signs of sepsis that are not commonly associated with IAA, such as fever, elevated white blood cell count, and positive blood cultures. If suspicion is high for the presence of an infected aneurysm, due to a history of bacteremia, endocarditis, IV drug abuse, or abdominal sepsis, a rigorous diagnostic effort should be initiated. At the minimum, tests would include multiple “downstream” arterial blood cultures and echocardiography. Confirmation of diagnosis pre-operatively is critical, as the operative strategy for infected aneurysms is markedly different from the interventions for inflammatory aneurysms.
Operative Considerations and Technique
At first it was commonly thought that the dense fibrosis associated with IAA would provide mechanical support to the aneurysm and thus decrease the incidence of rupture. This has not been confirmed by any series; therefore, the indications for repair are the same as those for other AAA. In the absence of obstructive gastrointestinal or urologic symptoms, patients with IAA can be followed unless the aneurysm exceeds 5.5 cm or diameter expands by more than 0.5 cm in 1 year. That said, many patients with inflammatory aneurysms are highly symptomatic with back pain, abdominal pain, or weight loss despite relatively small aneurysms. Because these symptoms can be incapacitating and cannot be reliably distinguished from impending rupture, repair is advisable. As in all patients with AAA, tenderness to palpation should lead to an urgent repair. Of course, any radiologic or clinical evidence of rupture is treated immediately, regardless of aneurysm size.
Preparation for elective operation is similar to that for repairs of other infrarenal aneurysms. While some surgeons order gentle bowel preparations pre-operatively, we do not routinely do this. Placement of ureteral stents is reserved for those few patients who present with both hydronephrosis and renal insufficiency secondary to obstruction. In this specific setting, optimizing renal function pre-operatively has significant benefit and should be achieved if time allows. In patients without clinically evident ureteral obstruction, the utility of this technique has not been proven. Just prior to the operation, the patient should receive prophylactic antibiotics per institutional protocol. Central venous monitoring should be instituted via triple lumen catheter or Swan-Ganz line, and an intra-arterial line should be placed. Four units of packed red blood cells should be readily available. Blood salvage and autotranfusion devices should be available to minimize the need for allogeneic blood transfusion.
Surgical Exposure
There is some debate about the best aortic exposure for repair of inflammatory aneurysms, with relatively equal enthusiasm expressed for both transperitoneal and retroperitoneal approaches. Practitioners of transperitoneal exposure like the familiarity of exposure of the infrarenal aortic neck and the easy access to the supraceliac aorta. Those that prefer the retroperitoneal approach note the improved access to the suprarenal aorta for cross clamping as well as the ability to avoid dense anterior aortic adhesions to the duodenum. Elevation of the left kidney during a retroperitoneal exposure also reduces injury to the left ureter during repair. The final decision relies on the experience and preference of the surgeon as well as information obtained from pre-operative CT scans, which may point to specific anatomic hazards of a particular approach. That said, we generally prefer a retroperitoneal approach if the diagnosis of IAA is recognized pre-operatively.
Regardless of the surgical exposure, the key to safe repairs of inflammatory aneurysms is minimal peri-aortic dissection. Dense adhesions between the duodenum, vena cava, renal vein, ureters, and retroperitoneum can complicate exposure of the infrarenal aortic neck and predispose to injury to these structures. In particular, dissection of the third and fourth portion of the duodenum away from the aorta should be avoided, as this maneuver is likely to be unsuccessful, and the adverse sequelae of enterotomy are substantial. Typically, the dense fibrosis is limited to the aorta caudal to the renal vein, and often a safe area for dissection and aortic control can be found just above this level.
If the transperitoneal approach is used, an intra-operative decision must be made regarding the proximal extent of the inflammation. In some cases merely dividing the gonadal vein may aid in retraction of the left renal vein to expose an uninvolved aortic neck. Alternatively, the left renal vein may be divided to allow for better aortic exposure if the gonadal and lumbar tributaries have been preserved. If the juxtarenal aorta is also involved in the inflammatory process, supraceliac clamping may be necessary. Access is best gained by dividing the gastrohepatic ligament and retracting the esophagus and proximal stomach to the patient’s left and dividing the diaphragmatic crus. Identification of the esophagus is aided by placement of a bougie or nasogastric tube. It is not necessary to encircle the aorta at this point, although anterior and both lateral aspects should be adequately dissected in the mediastinum just proximal to the celiac axis.
Retroperitoneal exposure with elevation of the left kidney, pancreas, and spleen affords complete and continuous access to the aorta. The most distal yet safe location for aortic clamping can be precisely determined. In our experience, clamping above the renal arteries (either suprarenal or supraceliac) is needed in about 75% of patients.
Once proximal aortic control has been achieved, the aorta is incised longitudinally along the left anterolateral surface to avoid injury to the duodenum. It is important to be aware of the left ureter at this point. Unlike most atherosclerotic aneurysms, where the ureters are pushed laterally, in IAA the ureters are drawn centrally by the fibrosis and can be injured during aortotomy. Endoluminal control of the iliac arteries is preferred using balloon occlusion. A tube graft is used for reconstruction if at all possible. A larger needle with stout 2-0 or 3-0 monofilament suture is used with generous tissue bites and endoaneurysmorraphy technique. After completing the proximal anastomosis, the clamp on the suprarenal aorta should be removed and replaced on the graft. If tunneling to the right groin for a femoral anastomosis is required for reconstruction, extreme caution should be exercised; in these unusual circumstances, consideration should be given to a direct passage to the left groin through the retroperitoneal exposure with a left to right femoral-femoral bypass.
Postoperative Complications Specific to Inflammatory Aneurysms
Some studies have demonstrated a statistically increased incidence of renal failure in patients after repair of IAA. This is likely secondary to pre-operative obstructive uropathy and the frequent need for suprarenal cross clamping. Selective pre-operative urinary
decompression and expeditious performance of the proximal anastomosis are the best steps to prevent this complication.
decompression and expeditious performance of the proximal anastomosis are the best steps to prevent this complication.
Inadvertent duodenal injury occurs rarely but is catastrophic especially if unrecognized. Prompt repair is always indicated; consideration of a partially diverting gastrojejunostomy is appropriate. If ureteral injury occurs from direct trauma or devascularization, nephrostomy tube drain or nephrectomy is needed.
One study has also shown an increased incidence in late para-anastomotic pseudo-aneurysm formation in these patients. This has not been our experience, but the relatively small numbers of these patients belies the statistical power of any individual institution’s perspective. Postoperative duplex or CT scanning may be warranted, though it is not our practice to do this routinely. In the majority of patients, the retroperitoneal fibrosis will regress after successful repair. Those who have continued ureteral entrapment and obstruction may require subsequent decompression. There is no evidence that the treatment of these patients with anti-inflammatory medication is therapeutic.
Venous Anomalies
While venous anomalies are relatively rare, occurring in only 2% of candidates for aortic aneurysm repair, inadvertent injury of these vessels can have devastating consequences. Pre-operative identification and understanding of the most frequent venous anomalies are important to prevent untoward and potentially lethal intra-operative events.
The embryologic development of the venous system occurs in a series of modifications of venous return throughout gestation that are characterized by appearance and regression of postcardinal, subcardinal, and ultimately, supracardinal veins. Retention of primitive anatomy in less than 10% of instances creates a wide range of venous anomalies from the presence of a retroaortic left renal vein to complete transposition of the inferior vena cava (IVC).
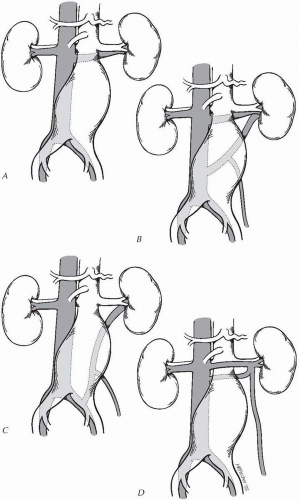
In practical terms, venous anomalies can be most simply divided into two groups, those associated with an aberrant left renal vein and those associated with a left-sided IVC. The retroaortic left renal vein has three main variations (Fig. 19-1). The type I retroaortic left renal vein lies in the retroaortic position at the level of the renal arteries. In this anomaly, the anterior component of the left renal vein has regressed. The incidence in the general population ranges from 0.3% to 1.9%. The type II retroaortic left renal vein drains caudally on the IVC or directly into the left iliac vein. In distinction from the type I left renal vein, the type II left renal vein will cross the aorta posteriorly at about the level of the inferior mesenteric artery IMA. The incidence in the
general population ranges from 0.4% to 0.9%. The third type of venous anomaly is the circumferential left renal vein or “venous collar.” Persistence of the subcardinal and supracardinal veins results in a range of anomalies from a lattice of small retroaortic veins that empty into the IVC to the presence of a true aortic collar with both an anterior and a posterior left renal vein. The incidence in the general population of such a venous ring is about 2%.
general population ranges from 0.4% to 0.9%. The third type of venous anomaly is the circumferential left renal vein or “venous collar.” Persistence of the subcardinal and supracardinal veins results in a range of anomalies from a lattice of small retroaortic veins that empty into the IVC to the presence of a true aortic collar with both an anterior and a posterior left renal vein. The incidence in the general population of such a venous ring is about 2%.
There are two types of caval anomalies: duplication and transposition. The incidence of a double IVC varies from 0.2% to 3%. In this aberration, the duplicated cavae run parallel to the aorta. The left side drains into the left renal vein or crosses the aorta at the level of the renal arteries anteriorly or, less often, posteriorly. In complete duplication, communication between the iliac vein and right IVC is maintained such that the left IVC can be ligated for exposure of the juxtarenal aorta. In transposition, a large single IVC runs along the left side of the aorta and crosses to the right side at the level of the renal arteries, where it continues proximally like a right-sided IVC. Typically the gonadal and adrenal veins form a reverse image of the normal anatomy draining into the renal vein on the right and directly into the IVC on the left. The incidence of caval transposition in the general population is 0.2% to 0.5%. An additional anomaly, anterior left iliac vein, should also be noted. This often occurs in conjunction with a retrocaval or retroiliac ureter.
Diagnosis and Pre-operative Assessment
While the incidence of intra-abdominal venous anomalies in the general population may be as high as 5%, the incidence of retroaortic left renal vein in patients who require aortic reconstruction is less than 2% in most series. That said, the incidence of venous injury in these patients has been reported as high as 40%. The seriousness of these injuries cannot be understated and mandates that the operating surgeon identify the anatomy pre-operatively. Routine and systematic review of pre-operative CT scans for the presence of a venous anomaly is the most important precaution (Fig. 19-2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree