Small Cell Carcinoma
Allen P. Burke, M.D.
Definition and Terminology
Small cell lung carcinoma (SCLC) is a high-grade, highly proliferative carcinoma with characteristic histologic features and frequent endocrine differentiation. It is most common in the lung, but occurs in virtually every organ that may develop epithelial malignancy. The term “small cell carcinoma” is the preferred term for carcinomas with areas of classic morphology. Although 5% to 7% of cases may be difficult to separate from non-small cell carcinoma on routine microscopy, the use of “intermediate” or “gray-zone” categories has been discouraged.1 The term “combined small cell carcinoma” is used if there are areas of other carcinoma types.
Epidemiology
The proportion of lung cancers that are small cell lung carcinomas has declined over the past several decades, from about 20% in the late 1990s to 15% of primary lung cancers today; this is attributed to the concurrent decrease in smoking. Small cell carcinoma is more highly associated with smoking than are adenocarcinomas, as 98% of small cell cancer patients are smokers.2 The relative risk for a man smoking >30 cigarettes daily is 111.3 times that of a nonsmoker compared to 103.5 for squamous carcinoma and 21.9 for adenocarcinoma.3 The decrease in rate of small cell carcinoma has been proportionately less in women and nonwhites.4,5
There is no gender bias for small cell carcinoma. A high proportion of patients (60%) present with advanced disease (stage IV).4
Staging
Radiologic Findings
Small cell lung cancers are central lesions, most presenting with hilar or mediastinal lymph node metastases. Only 5% occur as a peripheral solitary coin lesion.7 The mass is often lobulated and is less frequently endobronchial than squamous carcinomas and only rarely cavitary. Compared to other primary lung cancers, invasion of hilar vessels and of the superior vena cava is relatively common.
Tissue Sampling
Almost all patients with small cell carcinoma are diagnosed on cytologic preparations, which include smears and cell blocks, on which immunohistochemical preparations can be done. Only 3.5% of cancer resections of the lung reveal pure small cell carcinoma.8 Because small cell carcinomas are usually central lesions with positive lymph node disease, diagnostic procedures include, in addition to endoscopic or transbronchial biopsy, needle aspiration cytology from either pulmonary endoscopy (endobronchial ultrasound [EBUS] guided) or esophageal endoscopy (endoscopic ultrasound [EUS] guided). In addition to establishing a diagnosis, lymph node biopsy by these techniques also confirms clinical staging by CT and/or PET/CT scans.
Gross Findings
Small cell carcinoma typically has a tan, homogeneous, soft to rubbery cut surface, often with necrotic areas. Cavitation and hemorrhage are not generally present.
Microscopic Findings
Small cell carcinoma is defined by histologic features present on hematoxylin-eosin stains (Table 79.1). Although immunohistochemical stains are often very helpful, their results can be misleading, because
some tumors fail to express any markers other than pan-cytokeratin, and expression is often patchy. On routine stains, cells are small, generally under 25 microns in diameter, round, oval, or slightly spindled, with finely granular nuclear chromatin, scant cytoplasm, and inconspicuous nucleoli (Figs. 79.1, 79.2, 79.3). There is no clear definition of “inconspicuous,” although plainly eosinophilic nucleoli should be absent or only occasional. Typically, there is nuclear molding (Figs. 79.4 and 79.5). Necrosis is common, and mitotic activity is high, with at least one per high-powered (40×) field (Figs. 79.6 and 79.7). Mitotic figures may be difficult to identify on cytologic samples, however, and the presence of apoptotic debris is a reasonable substitute, unless Ki 67 staining is performed, which should show a proliferative index of at least 50%. Multinucleated cells with minimal cytoplasm may be interspersed among otherwise typical small cell carcinoma (Fig. 79.8). Growth patterns of neuroendocrine differentiation including rosettes, peripheral palisading, organoid nesting, and trabeculae may occur in small cell carcinoma (Fig. 79.9) and do not exclude the diagnosis provided that cytologic features typical of small cell carcinoma are present. If there are prominent nucleoli and larger cells, then these features define large cell neuroendocrine carcinoma. When present in at least 5% to 10% of cells, then the diagnosis is combined small cell and large cell neuroendocrine carcinoma.
some tumors fail to express any markers other than pan-cytokeratin, and expression is often patchy. On routine stains, cells are small, generally under 25 microns in diameter, round, oval, or slightly spindled, with finely granular nuclear chromatin, scant cytoplasm, and inconspicuous nucleoli (Figs. 79.1, 79.2, 79.3). There is no clear definition of “inconspicuous,” although plainly eosinophilic nucleoli should be absent or only occasional. Typically, there is nuclear molding (Figs. 79.4 and 79.5). Necrosis is common, and mitotic activity is high, with at least one per high-powered (40×) field (Figs. 79.6 and 79.7). Mitotic figures may be difficult to identify on cytologic samples, however, and the presence of apoptotic debris is a reasonable substitute, unless Ki 67 staining is performed, which should show a proliferative index of at least 50%. Multinucleated cells with minimal cytoplasm may be interspersed among otherwise typical small cell carcinoma (Fig. 79.8). Growth patterns of neuroendocrine differentiation including rosettes, peripheral palisading, organoid nesting, and trabeculae may occur in small cell carcinoma (Fig. 79.9) and do not exclude the diagnosis provided that cytologic features typical of small cell carcinoma are present. If there are prominent nucleoli and larger cells, then these features define large cell neuroendocrine carcinoma. When present in at least 5% to 10% of cells, then the diagnosis is combined small cell and large cell neuroendocrine carcinoma.
TABLE 79.1 Cytologic Features Distinguishing Small Cell from Large Cell Neuroendocrine Carcinoma | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Crush artifact, especially on cytologic samples, is very common and often necessitates immunohistochemical staining for further characterization. Encrustation of vessel walls by basophilic degenerated nuclear debris from necrotic tumor cells may be seen and is termed the “Azzopardi effect.”1
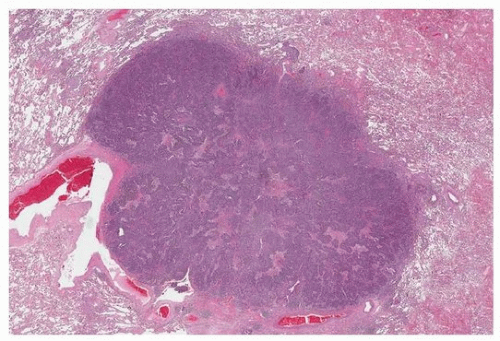 FIGURE 79.1 ▲ Small cell carcinoma. Low magnification demonstrating relatively circumscribed homogeneous cellular tumor. |
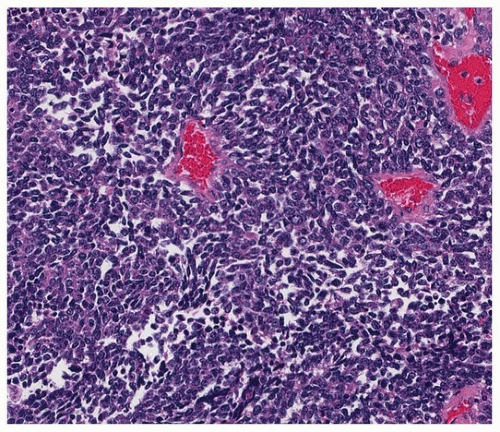 FIGURE 79.2 ▲ Small cell carcinoma. There is typical small fusiform “oat cell” morphology in this case, with barely discernable cytoplasm and dispersed nuclei without nucleoli. |
Neuroendocrine architectural patterns are especially frequent in resected specimens. Other histologic findings in wedge biopsies or lobectomies that differ from small samples include relatively infrequent crush artifact, slightly larger cell size, occasional prominent nucleoli, and vesicular nuclear chromatin.9
Immunohistochemical Stains
Endocrine Markers
In contrast to large cell neuroendocrine carcinomas, there is often spotty staining for endocrine markers in small cell carcinoma, with 10% expression generally considered positive.10 Because of the focal nature of staining, there is a wide range in the reported rate of positivity for endocrine markers (Table 79.2).
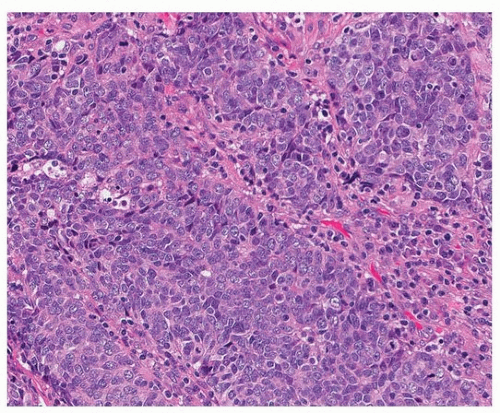 FIGURE 79.3 ▲ Small cell carcinoma. In this tumor, there are slightly larger cells with some cytoplasm, but dispersed nuclear chromatin and inconspicuous nucleoli are typical. |
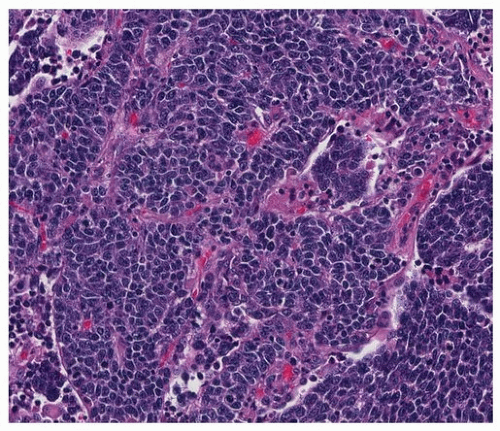 FIGURE 79.4 ▲ Small cell carcinoma. In this example, nuclear molding is prominent, and there is a slightly organoid growth pattern. |
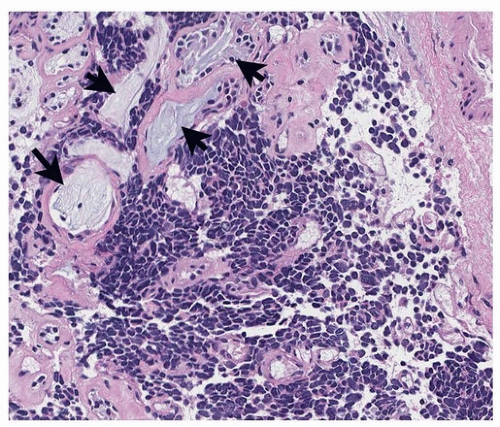 FIGURE 79.5 ▲ Small cell carcinoma. This tumor has the typical small, ovoid cellular structure and invades among mucous glands (arrows) in the bronchial wall. |
 FIGURE 79.7 ▲ Small cell carcinoma. Necrosis is typical in small cell carcinoma, in which cell outlines are still visible. |
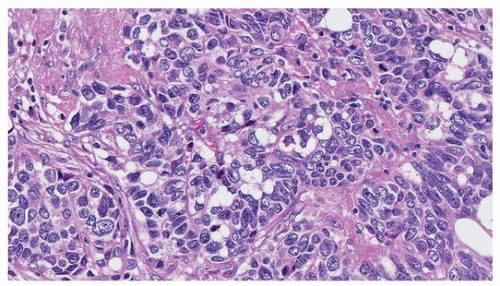 FIGURE 79.9 ▲ Small cell carcinoma. Crush artifact renders diagnosis difficult and may mimic lymphoid lesions, low-grade endocrine tumors, and other high-grade carcinomas. This area is a different one sampled from the tumor shown in Figure 79.8, where there was no crush artifact. |
TABLE 79.2 Immunohistochemical Findings in Pulmonary Small Cell Carcinoma | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




