Chapter 29 Small Bowel Transplantation
History
Disease processes that lead to an inability to sustain oneself via normal enteral nutrition remain a therapeutic challenge to patients and physicians alike. The terms short gut syndrome and short bowel syndrome are often used to describe patients who are dependent on total parenteral nutrition (TPN) because of significant loss of bowel length from a variety of causes, ranging from congenital malformations to traumatic injury to ischemic loss. Prior to the availability of TPN, short bowel syndrome was almost always fatal and, even with current therapies, extremely short remnant bowel length, less than 50 cm of jejunoileum in adults, is associated with a 43% 5-year mortality.1 However, the term short bowel syndrome excludes a subset of patients who may have a normal or near-normal intestinal length. Inflammatory illnesses such as Crohn’s disease, motility disorders such as intestinal pseudo-obstruction, and diseases of the enterocytes such as intestinal epithelial dysplasia share the same devastating consequence of being unable to sustain oneself via enteral means. For this reason, the term intestinal failure represents a more accurate and fully inclusive term when describing patients who are unable to tolerate enteral feedings.
Dudrick and associates’ seminal work2 in the laboratory showing that puppies could achieve near-normal growth patterns while exclusively sustained via hyperalimentation was one of the most significant medical breakthroughs of the century. Long-term TPN support increased patient survival, but also introduced a new set of problems, including potentially life-threatening infections and technical difficulties for maintaining access. As more patients were sustained for longer periods with TPN, cholestasis leading to liver failure was increasingly recognized as a potentially fatal complication.3 Until recently, transplantation or death appeared to be inevitable once a patient developed parenteral nutrition–associated liver disease (PNALD). However, a new investigational fish oil–based lipid formulation has been reported to prevent or even reverse TPN-induced cholestasis,4 and may be the next major breakthrough in this field.
Early investigational models of intestinal transplantation were being developed long before the advent of TPN, but were doomed to failure because of a lack of understanding of immunology. The first investigation of intestinal transplantation as novel therapy for intestinal failure is attributed to Alexis Carrel in 1905.5 Approximately 50 years later, in 1959, after the earliest successful kidney transplantation in Boston, Lillehei and colleagues6 at the University of Minnesota published their successful work transplanting intestines using a canine model. Starzl and associates7 in Pittsburgh reported transplanting a cluster of organs, including the intestine, in dogs in 1960. The first published human intestinal transplantation was performed by Lillehei and coworkers8 in 1967, but the patient unfortunately did not survive because of thromboembolic complications. Over the next few years, there were several other unsuccessful attempts in humans,9 primarily because of the inability to control rejection, resulting in overwhelming infections. Given the apparent success of parenteral nutritional support, there was diminished enthusiasm for further clinical trials of intestinal transplantation.
The advent of cyclosporine immunosuppression in the early 1980s coincided with descriptions of life-threatening complications associated with long-term TPN and led to renewed interest in the field of intestinal transplantation. Cohen and coworkers10 in Toronto reported the first isolated intestinal transplantation using cyclosporine in 1985 but, unfortunately, the 26-year-old recipient died on postoperative day 9. Over the next 5 years, several successful intestinal transplants procedures were performed by Deltz,11 Starzl,12 and colleagues, resulting in patient survival ranging from 6 months to longer than 20 years.13 In the early 1990s, substantial improvements in control of rejection accompanied the introduction of tacrolimus immunosuppression, and individual centers were able to demonstrate consistent successes.14,15 In the United States, over 1800 intestinal transplantation were performed through the end of 2009, based on Organ Procurement and Transplantation Network (OPTN) data (http://optn.transplant.hrsa.gov).
Nontransplantation Therapies for Short Bowel Syndrome
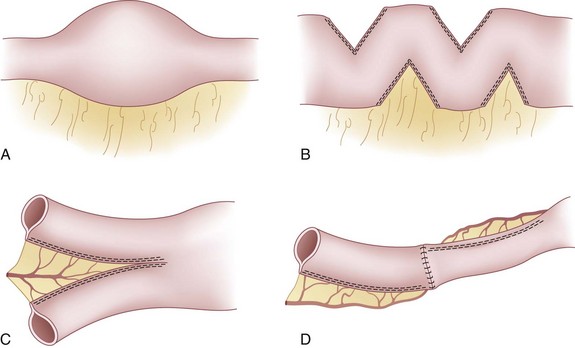
Although nontransplantation surgery is not the focus of this chapter, several novel surgical approaches were developed with the goal of maximizing function of the remaining intestine in short bowel syndrome. These surgical therapies play an important role in the history of the treatment of intestinal failure and are therefore mentioned briefly here. As early as the 1950s, the surgical creation of segments of antiperistaltic loops of bowel was proposed in an attempt to slow transit time in patients with limited intestinal length.16 Subsequently, it was recognized that the normal adaptive response to short bowel syndrome is to develop gradual dilation of the remnant segment, but this can lead to substantial dysmotility and bacterial overgrowth. Over time, this practice has been abandoned because of resulting dysmotility and bacterial overgrowth in these antiperistaltic loops. Tapering of these dilated bowel segments by excision of a portion of the bowel along the antimesenteric border to produce a more normal diameter can then improve forward motion of chyme, thus avoiding the need for resection of the dysmotile segment and further loss of length.17 In 1980, Bianchi18 described a novel alternative to resection and simple tapering consisting of separating distended bowel loops longitudinally so that two smaller diameter loops of bowel are created and reanastomosed in an isoperistaltic fashion. The resulting bowel is twice the length, but with only half of the original diameter (Fig. 29-1). Given the technical challenges involved, when more potent immunosuppression became available, attention was largely refocused on intestinal transplantation.
Kim and coworkers19 in Boston developed a technically simpler nontransplantation alternative to the longitudinal tapering and lengthening called serial transverse enteroplasty (STEP). This technique is performed by alternate staple firings from opposite directions across the distended loops of bowel in a zigzag pattern, resulting in a decreased final bowel diameter that allows for more normal motility and absorption (see Fig. 29-1). An International STEP Data Registry has been created to allow for the assessment of long-term follow-up of the efficacy and safety of the STEP procedure; the most recent publication has described the results in the first 38 patients undergoing the procedure at 19 centers.20 Another large single-center series has described 21 additional patients, which along with the registry demonstrate that application of STEP in carefully selected intestinal failure patients can avoid the need for transplantation in 50% to 60% of cases.21 Furthermore, in contrast to the Bianchi lengthening, STEP can be applied as a secondary lengthening procedure after primary Bianchi or STEP, with success rates similar to those after the initial lengthening.22 Clearly, a multidisciplinary approach with expertise in TPN management, nontransplantation surgical procedures, and intestinal transplantation is necessary to optimize care of the individual patient with intestinal failure.
Indications for Intestinal Transplantation
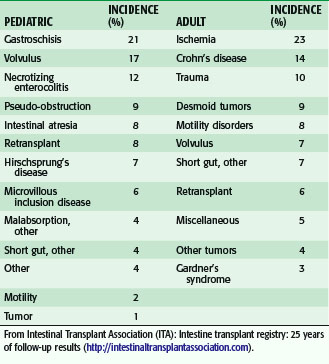
The underlying causes of intestinal failure in pediatric patients referred for intestinal transplantation are more likely to result from loss of length caused by congenital diseases when compared with adult patients (Table 29-1). The Intestinal Transplant Registry (ITR) has reported the three most common underlying disease states leading to transplantation in children as gastroschisis (21%), volvulus (17%), and necrotizing enterocolitis (12%).23 In contrast, the most common indications for intestinal transplantation in adults are ischemia (23%), Crohn’s disease (14%), and trauma (10%). Although the most common indications fall under the category of short bowel syndrome, children and adults may suffer from diseases that result in dysmotility or malabsorption, resulting in poor enteral function. For patients with short bowel syndrome, the remnant intestinal length and presence or absence of an ileocecal valve have been identified as predictive factors as to whether rehabilitation will be successful.24
Dependence on parenteral nutrition alone, however, is not considered an indication for intestinal transplantation. To be considered for intestinal transplantation, patients must be dependent on TPN and have experienced life-threatening complications of hyperalimentation. As early as 2000, the Center for Medicare and Medicaid Services recognized the following indications for transplantation of the intestine, with or without other organs, as standard of care for patients with irreversible intestinal failure: (1) overt or impending liver failure caused by PNALD; (2) multiple thromboses of central veins limiting central venous access; (3) more than two episodes of catheter-related infection requiring hospitalization in any year; (4) a single episode of fungal line infection; and/or (5) frequent severe dehydration, despite IV fluid supplementation and TPN. Chronic line infections, specifically fungal line infections, can be difficult to clear and can lead to venous occlusion, loss of available access sites, or death. Thrombosis can also occur outside the setting of infection, leading to loss of potential central venous access sites. In addition, approximately 50% of all patients on TPN will develop PNALD,25 thus potentially requiring liver transplantation in addition to intestinal transplantation.
In 2001, the American Society of Transplantation issued a position paper concerning indications for pediatric intestinal transplantation. In addition to the criteria noted, they also proposed that intestinal transplantation be considered for patients with intestinal failure that almost always results in early death, despite optimal nutrition, and for patients with high morbidity, poor quality of life, or fluid or electrolyte abnormalities who cannot be successfully managed on an outpatient basis.26 Despite establishment of these criteria a decade ago, many referrals for intestinal transplantation continue to occur late in the patient’s clinical course. Currently, 75% of patients on the waiting list for intestinal transplantation also require a liver, thus placing more strain on an already limited organ supply.27
Evaluation
Recipient Evaluation
After referral to an intestinal transplantation center, the patient undergoes evaluation to determine the extent of complications of intestinal failure and degree of comorbidities. Historical details and previous hospital records pertaining to surgical procedures performed, previous intestinal rehabilitation attempts, and attempts at enteral feeding will be closely examined. Common diagnostic studies performed during the evaluation are listed in Table 29-2. Once a patient is deemed an appropriate candidate, he or she is placed on the waiting list within their United Network for Organ Sharing (UNOS) region. Transplantation centers may indicate weight and age spans for which they would consider potential donors, and each patient is assigned a status level according to her or his available venous access. Level 1 recipients are those who no longer have adequate central venous access via the jugular, subclavian, or femoral vein and all others are classified as level 2 candidates. Potential recipients who also require a liver in addition to the intestine, are placed on the liver waiting list according to their model for end-stage liver disease (MELD) or pediatric end-stage liver disease (PELD) score (OPTN policy 3.11 on intestinal organ allocation).
Table 29-2 Some Diagnostic Studies for Pretransplantation Evaluation
| Diagnostic Studies | Examples |
|---|---|
| Laboratory evaluation | Serum chemistries, liver function tests, complete blood count, prothrombin time–international normalized ratio (PT-INR), partial thromboplastin time (PTT), platelet count, albumin, prealbumin |
| Serologies for infectious diseases | CMV, EBV, hepatitis panel, HIV |
| Endoscopy | Upper gastrointestinal (UGI) series, colonoscopy with biopsies |
| Pathology | Percutaneous liver biopsy |
| Radiographic evaluation | UGI with small bowel follow-through, CT of abdomen and pelvis, Doppler ultrasonography or magnetic resonance venography |
Donor Evaluation
Potential cadaveric intestine donors are matched to appropriate size- and blood type–compatible recipients according to UNOS policies—first locally, then regionally, and finally nationally. When centers are evaluating a potential donor, consideration is given to existing intestinal pathology or past surgical history, such as extensive resection or Roux-en-Y reconstruction. Donors may be deemed inappropriate based on Epstein–Barr virus (EBV) and cytomegalovirus (CMV) serologies, which could lead to post-transplantation lymphoproliferative disorder and severe enteritis in the recipients, respectively. Another consideration is the size of the donor compared with the recipient, because significant loss of abdominal domain after extensive resection may not allow accommodation of organs from larger or even size-matched donors. In these cases, an ideal donor should have a weight 50% to 75% that of the recipient. Furthermore, long cold ischemia times of the intestinal allograft may lead to loss of mucosal integrity and bacterial translocation or intestinal perforation in the donor organ. Therefore, consideration is given to any factor that could potentially increase the cold ischemia time, such as prior abdominal surgery in the donor and travel time between the recipient and donor medical centers. Close communication must occur between the donor and recipient teams to optimize timing. As with all cadaveric organs, the final evaluation occurs during the procurement operation, during which the intestine is closely inspected for anatomic or perfusion defects that might preclude its use. Overall, intestine is recovered from potential donors less frequently than any other solid organ, but rates of recovery have been increasing over time.28
Anatomic Considerations
Decisions as to which organs should be included are made based on the recipient’s underlying disease process and vary slightly based on individual center experience. Some centers have included stomach in the allograft for recipients suffering from gastroparesis preoperatively, with good functional results, whereas other centers have reported that the transplanted stomach may also suffer from gastroparesis.29 Historically, the right and transverse colon, which receive their arterial supply based on the superior mesenteric artery, were included as part of the intestinal transplant. The colon was placed orthotopically and anastomosed to the recipient colon or brought out as an end colostomy. An early series from Pittsburgh has described increased risk of graft loss14 and the practice was largely abandoned. Recent reports dispute this finding and a few centers now include the colon routinely.30
Surgical Technique
Isolated Intestinal Transplantation
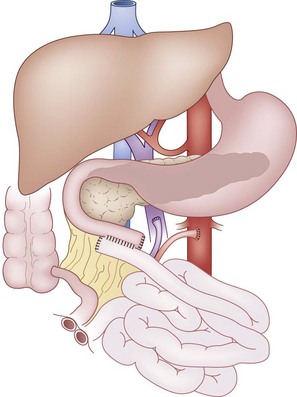
In adult and older pediatric donors without significant aberrations in anatomy, isolated intestine can be safely procured while still allowing for use of the liver and pancreas from the same donor.31 During the donor operation for an isolated intestinal graft, the jejunum is divided at the ligament of Treitz and the ileum at its terminus. After a careful dissection of the mesentery from the retroperitoneal organs and colon and systemic flushing with preservation solution, the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) are divided at the mesenteric root just distal to the middle colic vessels. In the neonatal donor, or other situations in which the isolated pancreas is not placed separately for transplantation, the SMA is divided at the level of the aorta and the portal vein is divided at the superior border of the pancreas to provide longer vessels to the intestinal allograft. Carotid or iliac arteries and iliac or jugular veins are also procured from the cadaveric donor to allow for vascular reconstruction. During the recipient operation, arterial inflow is established by direct anastomosis of the donor SMA to the recipient infrarenal aorta or by interposition of a donor arterial conduit. Venous outflow from the allograft is provided by anastomosis of the donor SMV to the recipient portal vein or inferior vena cava, with or without an interposition of donor venous conduit. The continuity of the bowel is established proximally and distally using standard techniques for enteric anastomoses. Finally, a distal ileostomy is created to allow for routine monitoring of the graft (Fig. 29-2).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree