Sleep disordered breathing (SDB) is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease; however, the contribution of SDB to incident heart failure (HF), coronary artery disease (CAD), and atrial fibrillation (AF) in patients with T2DM is unknown. We followed up 834 consecutive asymptomatic patients with T2DM (age 56 ± 11 years, 369 women) with normal exercise echocardiographic findings for ≤8 years using electronic health records. The demographics, cardiac risk factors, symptoms, diagnoses, and medications were collected at the echocardiography and validated from the electronic health records. SDB was confirmed by a comprehensive sleep evaluation and/or polysomnography before echocardiography. SDB was diagnosed in 188 patients (21%) at baseline; 116 were untreated. During a median follow-up of 4.9 years (interquartile range 3.9 to 6.1), 22 congestive HF, 72 CAD, and 40 AF incident events were observed. In the Cox proportional hazards models, SDB was associated with incident CAD (hazard ratio 1.8, 95% confidence interval 1.1 to 3.0, p = 0.01; adjusted hazard ratio 1.9, 95% confidence interval 1.2 to 3.2, p <0.01) and AF (hazard ratio 2.6, 95% confidence interval 1.4 to 4.7, p = 0.01; adjusted hazard ratio 2.9, 95% confidence interval 1.5 to 5.9, p <0.01). Limiting SDB to only those patients diagnosed using polysomnography (n = 132), SDB was associated with incident CAD (hazard ratio 1.9, 95% confidence interval 1.1 to 3.3, p = 0.03; adjusted hazard ratio 2.2, 95% confidence interval 1.2 to 3.9, p = 0.01) and HF (hazard ratio 2.7, 95% confidence interval 1.1 to 7.0, p = 0.03; adjusted hazard ratio 3.5, 95% confidence interval 1.4 to 9.0, p <0.01). Female gender, age, elevated blood pressure, and left ventricular mass were additional correlates of CAD in those with asymptomatic T2DM. In conclusion, the association of SDB with incident CAD, AF, and HF in patients with T2DM justifies more liberal screening for SDB in patients with T2DM, realizing that SDB is a potentially modifiable risk factor.
The prevalence of patients with type 2 diabetes mellitus (T2DM) has reached epidemic proportions in the United States, involving 8.3% of the general population and 26.9% of patients aged ≥65 years. Adults with T2DM have a 2 to 4 times greater risk of heart disease-related mortality compared to their nondiabetic counterparts. Sleep disordered breathing (SDB) has been associated with T2DM and impaired glucose tolerance, independent of other co-morbidities, including obesity. Although a number of studies have assessed the association of SDB with cardiovascular events, none has focused on the effect of SDB on incident cardiovascular events in patients with asymptomatic T2DM without a history of cardiac disease. The aim of the present study was to define the role of SDB in incident heart failure (HF), coronary artery disease (CAD), and atrial fibrillation (AF) in patients with T2DM without these diagnoses and without evidence of subclinical CAD at baseline.
Methods
The present study was a longitudinal observational study of adult patients with T2DM without a known cardiovascular history, who had been referred for stress echocardiography to rule out CAD, in the absence of cardiac symptoms. The most common cause for testing was a cardiac risk evaluation, mainly before noncardiac surgery. The day of the echocardiogram was the entry date, and patients were followed up through October 31, 2011. Baseline data available from the Cleveland Clinic echocardiogram registry were supplemented by abstraction from the electronic medical records and manual verification of the administrative and polysomnography reports. The patient data were de-identified before statistical analyses. The institutional review board of Cleveland Clinic approved the study.
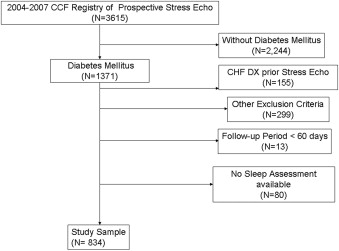
Exercise echocardiography was performed in 1,371 patients with T2DM aged ≥18 years, from January 1 2004 to December 31 2007. The exclusion criteria were a history of CAD, AF or atrial flutter, congestive HF, hypertrophic cardiomyopathy, valvular heart disease, aortic aneurysm, congenital heart disease, moderate or severe valvular heart disease, cardiac surgery, chronic pulmonary disease, neoplasia, human immunodeficiency virus, cirrhosis, kidney/heart transplantation and/or dialysis, positive exercise echocardiographic findings, ejection fraction of <50%, and a diagnosis of congestive HF, CAD, and/or AF within 30 days of the echocardiogram ( Figure 1 ). In addition, patients with a follow-up period of <60 days, those residing outside of Ohio, and patients with <1 comprehensive sleep evaluation within a 1-year period before the echocardiogram were excluded. Our final sample consisted of 834 patients.
The baseline variables consisted of age, gender, race/ethnicity, socioeconomic characteristics, cardiac risk factors, co-morbidities, current medications, laboratory values, weight, height, body mass index, heart rate, ejection fraction, and systolic and diastolic blood pressure. The socioeconomic characteristics were marital status, postal code, and county of residence; the latter 2 were used to estimate a “neighborhood” socioeconomic unit of analysis. The cardiac risk factors included age (men ≥50 years, women ≥60 years), hypertension, smoking, and a family history of CAD or low-density lipoprotein level of ≥100 mg/dl. The exercise echocardiograms were performed in the usual fashion. The Duke treadmill score was calculated using the symptom, electrocardiographic, and exercise responses to stress testing. Regional wall motion was assessed at rest and during stress. Only patients with normal left ventricular function at rest, no deterioration induced by stress, and a normal response in all segments were included in the present study.
SDB was considered present at baseline if identified before or within a maximum of 30 days after the baseline echocardiogram. Using the electronic medical record, we identified patients with SDB if the diagnosis was made using polysomnography in the presence of >5 apnea episodes, hypopnea episodes, or respiratory event-related arousals per hour in a symptomatic patient and >15/hour in an asymptomatic patient or if a comprehensive sleep evaluation identified the patient to be at high risk of SDB but the patient did not undergo polysomnography. Although polysomnography is the reference standard for the diagnosis of sleep disorders, assuming that patients without polysomnogram-confirmed SDB were negative for the disease would have underestimated SDB in our study population owing to patient nonadherence with testing. A subanalysis was conducted limiting patients diagnosed with SDB to those with abnormal polysomnographic findings. Patients were considered to not have SDB if they were found to be at low or no risk of having SDB using the comprehensive sleep evaluation or the sleep study findings were negative within 1 year of the echocardiogram.
Treatment of SDB was assessed for all patients, and adherence to treatment was measured by self-report. Patients who were prescribed continuous positive airway pressure or bilevel positive airway pressure but who reported therapy discontinuation, an inability to use the therapy, or poor or inadequate use were categorized as “untreated.” Because of the lack of objective data on the use of continuous positive airway pressure and bilevel positive airway pressure, no analyses were performed to compare patients with SDB who were treated versus untreated.
Patients were followed up for ≤7.8 years, and incident cardiac events were abstracted using the “International Classification of Diseases, 9th revision,” diagnostic codes. The patients were censored at HF or death. Mortality was determined using the Social Security Death Index obtained from the Ohio Department of Health; 13 patients were lost because of noncardiac-related death during follow-up, and death was not identified as an event.
The baseline differences were assessed according to SDB status using a chi-square test for categorical variables and a 2-sample t test for continuous variables. Differences between the Kaplan-Meier survival curves were compared using the log-rank test. Nested Cox proportional hazards models were used to assess the effect of SDB on the outcomes of interest, independent of other risk factors. Standard statistical software (SAS, version 9.2, SAS Institute, Cary, North Carolina) was used to perform the analyses, and p <0.05 was the cutoff for statistical significance.
Results
Our sample consisted of 834 patients with T2DM, without clinical evidence of CAD and negative baseline exercise echocardiographic findings, with a median follow-up of 4.9 years (interquartile range 3.9 to 6.1). The mean age was 56.2 ± 11.3 years, 19.5% of the patients were black, and 7.9% resided in areas with high poverty rates ( Table 1 ). The risk factors for cardiac complications were highly prevalent, and 79% had blood pressure levels greater than the 2011 American Diabetes Association recommended values. SDB was identified in 188 patients at baseline, 70% of whom were diagnosed using polysomnography, and 38% of whom used continuous positive airway pressure. As expected, patients with SDB had a significantly greater body mass index than patients without SDB ( Table 1 ).
| Baseline ∗ Characteristics | Total (n = 834) | SDB | p Value | |
|---|---|---|---|---|
| Yes (n = 188) | No (n = 646) | |||
| Women | 369 (44.2%) | 95 (50.5%) | 274 (42.4%) | 0.05 † |
| Black race | 163 (19.5%) | 38 (20.2%) | 125 (19.4%) | 0.08 |
| High poverty postal code | 66 (7.9%) | 8 (4.3%) | 58 (9.0%) | 0.03 † |
| Single | 122 (14.6%) | 29 (15.4%) | 93 (14.4%) | 0.73 |
| Body mass index ≥30 kg/m 2 | 475 (57.0%) | 148 (78.7%) | 327 (50.6%) | <0.0001 † |
| Body mass index ≥25 kg/m 2 | 731 (87.7%) | 182 (96.8%) | 549 (85.0%) | <0.0001 † |
| Hypercholesterolemia | 584 (70.0%) | 137 (72.9%) | 447 (69.2%) | 0.43 |
| Hypertension | 508 (60.9%) | 131 (69.7%) | 377 (58.4%) | 0.005 † |
| Cerebrovascular disease | 37 (4.4%) | 6 (3.2%) | 31 (4.8%) | 0.35 |
| Depression or anxiety | 201 (24.1%) | 57 (30.3%) | 144 (22.3%) | 0.02 † |
| Age (yrs) | 56.2 ± 11.3 | 54.6 ± 10.1 | 56.7 ± 11.6 | 0.024 † |
| Age ≥75 yrs | 48 (5.8%) | 5 (2.7%) | 43 (6.7%) | 0.04 † |
| Family history of coronary artery disease | 317 (45.1%) | 83 (44.2%) | 234 (36.2%) | 0.14 |
| Ever smoked | 376 (45.1%) | 90 (47.9%) | 286 (44.3%) | 0.38 |
| Coronary artery disease risk factors ‡ | 409 (49%) | 92 (48.9%) | 317 (49.1%) | 0.97 |
| Suboptimal blood pressure control § | 658 (78.9%) | 153 (81.4%) | 505 (78.2%) | 0.34 |
| Suboptimal glycemic control ¶ | 396 (47.5%) | 99 (52.7%) | 297 (46.0%) | 0.12 |
| Creatinine ≥1.5 mg/dl | 20 (2.4%) | 4 (2.1%) | 16 (2.5%) | NA |
| Low-density lipoprotein ≥100 mg/dl | 387 (46.4%) | 92 (48.9%) | 295 (45.7%) | 0.43 |
| Hemoglobin A1c (%) | 7.4 ± 1.7 | 7.4 ± 1.5 | 7.4 ± 1.8 | 0.97 |
| Glucose (mg/dl) | 144.2 ± 63.4 | 143.4 ± 52.9 | 144.4 ± 66.3 | 0.84 |
| Creatinine (mg/dl) | 0.88 ± 0.39 | 0.94 ± 0.69 | 0.86 ± 0.24 | 0.014 † |
| Low-density lipoprotein (mg/dl) | 104.2 ± 34.3 | 105.9 ± 33.7 | 103.7 ± 34.5 | 0.45 |
| High-density lipoprotein (mg/dl) | 52.0 ± 14.7 | 49.9 ± 12.4 | 52.6 ± 15.3 | 0.031 † |
| Triglycerides (mg/dl) | 166.4 ± 113.8 | 185.1 ± 125.9 | 160.8 ± 109.5 | 0.012 † |
| Body mass index (kg/m 2 ) | 32.1 ± 6.8 | 36.0 ± 7.3 | 30.9 ± 6.2 | <0.0001 † |
| Body surface area (m 2 ) | 2.0 ± 0.3 | 2.1 ± 0.2 | 2.0 ± 0.3 | <0.0001 † |
| Maximum heart rate (beats/min) | 154.8 ± 15.7 | 156 ± 14 | 154 ± 16 | 0.24 |
| Systolic blood pressure (mm Hg) | 134.0 ± 18.1 | 135 ± 18 | 134 ± 18 | 0.19 |
| Diastolic blood pressure (mm Hg) | 81.6 ± 9.8 | 83 ± 10 | 81 ± 10 | 0.06 |
| Ejection fraction (%) | 63.0 ± 7.8 | 62 ± 8 | 63 ± 8 | 0.04 † |
| Left ventricular mass (g) | 177.6 ± 56.5 | 189.2 ± 55.4 | 177.9 ± 56.4 | 0.005 † |
| Diastolic dysfunction stage | 0.16 | |||
| 1 | 362 (43.4%) | 74 (39.4%) | 288 (46.6%) | |
| 2 | 35 (4.2%) | 4 (2.1%) | 31 (4.8%) | |
| 3 | 5 (0.6%) | 1 (0.5%) | 4 (0.6%) | |
| End-diastolic volume (ml) | 85.8 ± 25.0 | 90.9 ± 26.6 | 84.3 ± 24.3 | <0.001 † |
| Indexed end-diastolic volume (ml) | 42.3 ± 10.7 | 42.4 ± 11.4 | 42.3 ± 10.4 | 0.93 |
| Median left atrial area (cm 2 ) | 16.2 ± 4.0 | 16.7 ± 3.9 | 16.0 ± 4.0 | 0.05 † |
| Abnormal Duke score | 103 (12.4%) | 19 (10%) | 84 (13%) | 0.29 |
| Insulin | 152 (18.2%) | 42 (22.3%) | 110 (17.0%) | 0.10 |
| β Blockers | 133 (16.0%) | 33 (17.0%) | 101 (15.6%) | 0.65 |
| Angiotensin-converting enzyme inhibitors | 311 (37.3%) | 80 (42.6%) | 231 (35.8%) | 0.09 |
| Diuretics | 285 (34.2%) | 79 (42.0%) | 206 (31.9 %) | 0.01 † |
| Aspirin | 315 (37.8%) | 68 (36.2%) | 247 (38.2%) | 0.61 |
| Statins | 465 (55.8%) | 108 (57.5%) | 357 (55.3%) | 0.60 |
| Nonsteroidal anti-inflammatory drug | 208 (24.9%) | 63 (33.5%) | 145 (22.5%) | 0.002 † |
| Continuous positive airway pressure or bilevel positive airway pressure at baseline | 72 (8.6%) | 72 (38.3%) | NA | NA |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


