Sleep Apnea Syndromes: Central and Obstructive
HISTORY OF SLEEP-DISORDERED BREATHING
Sleep-disordered breathing (SDB) is an extremely common medical disorder associated with important morbidity. Recognition of its relevance in medicine is relatively recent, although clinical reports of SDB were first made in the 19th century.1 Likely influenced by such observations, descriptions of an entity constituting obesity and extreme somnolence were highlighted in the character narratives of the “fat boy” in Charles Dickens’ series, Posthumous Papers of the Pickwick Club, first published in 1837.2 Dickens described Joe, the fat boy, as a loud snorer who was obese and excessively somnolent—the classical description of Pickwickian syndrome. Sir William Osler in 1918 was credited with first linking the relationship between obesity and Pickwickian syndrome.3 In the mid-20th century, further work led to the association of Pickwickian syndrome with alveolar hypoventilation by Burwell et al. in 1956, and periodic cessation of respiration by Drachman and Gumnit in 1962.4,5
Over the last 40 years, we have begun to understand the pathogenesis of sleep apnea and have developed effective diagnostic and treatment modalities for this common disorder. Gastaut et al.6 in 1965 showed that cessation of respiration was due to obstruction of the upper airway, and obstructive sleep apnea (OSA) was recognized. In 1972, a conference organized by Lugaresi and his Bologna (Italy) group entitled “Hypersomnia and Periodic Breathing,” served as a springboard for the growth of interest and research in SDB.7 Guilleminault et al.8 coined the terms sleep apnea syndrome and obstructive sleep apnea syndrome (OSAS) in 1976 to underscore that airway obstruction during sleep was not restricted to obese subjects.
As the understanding of the etiology of OSA increased, treatment options began to emerge. In 1969, a case report describing treatment of OSA in a patient with tracheostomy was published.9 The first reports demonstrating reversal of OSA with positive airway pressure (PAP) did not occur until more than 10 years later.10,11 The role of weight loss and its shortcomings in resolution of OSA was noted by Fishman in 1972.12
SLEEP APNEA DEFINITIONS
Adult SDB is present when repetitive apneas (episodes of breathing cessation) and hypopneas (episodes of decrement in airflow) occur during sleep, usually associated with sleep fragmentation, arousals, and reductions in oxygen saturation. Apneas are generally defined as an episode of breathing cessation lasting at least 10 seconds in duration, and can be classified as obstructive (in which there is no airflow despite continued respiratory effort), central (no airflow and no respiratory effort), or mixed (events initially appear central in origin, with respiratory effort occurring during the latter portion of the same episode). Hypopneas are SDB events during which decrements in airflow are observed and can also be obstructive or central in origin. In practice, hypopnea definitions vary substantially according to the degree of airflow limitation expected, the magnitude of oxygen desaturation needed, and by whether the definition includes events associated with arousal but not oxygen desaturation.13,14 In addition, the term “respiratory effort–related arousal” (RERA) is sometimes used to designate a series of breaths characterized by increasing effort leading to an arousal from sleep that does not meet the criteria for apnea or hypopnea.15 Although both physiologic and epidemiologic studies have demonstrated that hypopneas produce the same clinical consequences as apneas, evolving hypopnea definitions can make it challenging to compare sleep apnea severity and associated consequences across different study populations.
Currently, the apnea–hypopnea index (AHI, number of apneas and hypopneas per hour of sleep) is the standard metric used to describe the severity of sleep apnea.13 In older papers, the term respiratory disturbance index (RDI) was often used interchangeably with AHI. More recently, some authors have included the number of RERAs per hour of sleep in the RDI definition, that is, number of apneas, hypopneas, and RERAs per hour of sleep. Nevertheless, neither AHI nor RDI take into consideration other characteristics of SDB such as the magnitude of oxygen desaturation or duration of hypoxia.
 OBSTRUCTIVE SLEEP APNEA
OBSTRUCTIVE SLEEP APNEA
OSA is defined by the presence of repetitive episodes of upper airway obstruction during sleep. An AHI of equal to or greater than 5 events/h is commonly used to define OSA, with obstructive or mixed (rather than central) events comprising more than 50% of the total.14 The OSAS is usually defined by an AHI equal to or greater than 5 events/h and persistent complaints of excessive daytime somnolence, unrefreshing sleep, or fatigue.16 Other OSA definitions suggest that OSA be recognized if the AHI is at least 10 to 14 events/h, or in cases in which the AHI is between 5 and 14 events/h and documented hypertension, ischemic heart disease, history of stroke, or symptoms of excessive daytime sleepiness, impaired cognition, mood disorders, or insomnia exist.17,18
OSA is often classified as mild, moderate, or severe according to the AHI. A common scheme is 5 to 15 (mild), 15 to 30 (moderate), >30 events/h (severe).14 Some authors have suggested that severity should be defined by associations with adverse clinical outcomes rather than the event index.19,20 Recent changes in clinical hypopnea definitions by organizing bodies such as the American Academy of Sleep Medicine (AASM) have also provoked debate over parameters of disease severity.14,21,22
In the past, the term upper airway resistance syndrome (UARS) has been used to describe symptoms of sleepiness and fatigue in the setting of milder SDB not meeting criteria for apneas and hypopneas.23 Advances in detection of SDB events (e.g., with nasal pressure) and the inclusion of events associated with arousals in the hypopnea definition have likely eliminated the need to designate UARS as a separate entity.14,24 These individuals are now likely to be diagnosed with OSA. Debate continues as to whether some nonobese individuals may have a hypersensitive arousal response to repetitive increased respiratory effort that is distinct from OSA.25,26
 CENTRAL SLEEP APNEA
CENTRAL SLEEP APNEA
Central sleep apnea (CSA) is much less common than OSA and is characterized by a transient cessation of rhythmic breathing. It is defined as repeated episodes of apnea in the absence of respiratory muscle effort and is observed on the polysomnogram as an absence of both nasal–oral airflow and thoracoabdominal excursion.27 A number of etiologies for CSA have been recognized, among which congestive heart failure (CHF) and stroke are the most common. Patients with CSA experience sleep fragmentation and can report similar daytime symptoms to OSA patients.
 OBESITY HYPOVENTILATION SYNDROME
OBESITY HYPOVENTILATION SYNDROME
Obesity hypoventilation syndrome (OHS), or the Pickwickian syndrome, is defined by obesity, SDB, and hypoventilation with daytime hypercapnia in the absence of other causes for hypoventilation. Thus, OHS should be regarded as a diagnosis of exclusion. Seventy to 90% of patients with OHS have been estimated to have OSA.28,29 Conversely, 10% to 15% of sleep apnea patients have been noted to have daytime hypercapnia and can be classified as having concomitant OHS.30 OHS is discussed more fully in Chapter 100.
PATHOGENESIS OF OBSTRUCTIVE SLEEP APNEA
The pathogenesis of OSA involves both anatomic and neurologic components. The upper airway is a complicated structure that performs multiple physiologic functions, including vocalization, respiration, and deglutition. The upper airway extends from the posterior margin of the nasal septum to the larynx and has a paucity of rigid bony support. It is divided into four anatomic regions (Fig. 99-1):
• Nasopharynx: between the nares and the hard palate
• Retropalatal oropharynx: between the hard palate and the caudal margin of the soft palate
• Retroglossal oropharynx: between the caudal margin of the soft palate and the base of the epiglottis
• Hypopharynx: from the base of the tongue to the larynx
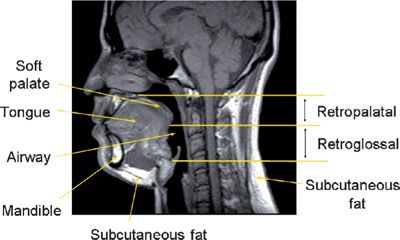
Figure 99-1 Midsagittal magnetic resonance image in a normal individual demonstrating the anatomic regions of the upper airway and relevant craniofacial and soft tissue structures. The retropalatal (RP) region is defined from the level of the hard palate to the distal margin of the soft palate; the retroglossal (RG) region is defined from the distal margin of the soft palate to the base of the epiglottis. In patients with OSA, obstruction usually occurs in the retropalatal or retroglossal levels or at both locations. (Reproduced with permission from Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–1689.)
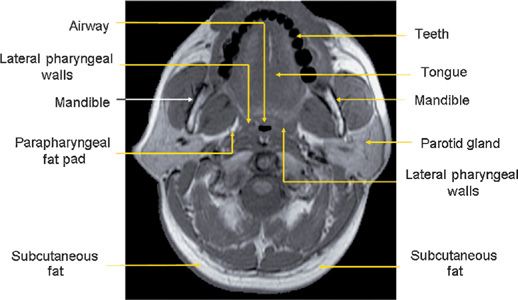
The major contributors to the airway boundaries include the soft palate and tongue anteriorly; the pharyngeal constrictor muscles, lymphoid tissue, parapharyngeal fat pads, and mandibular rami laterally; and the pharyngeal constrictor muscles posteriorly.31,32 The soft tissue and craniofacial (bony) structures in the retropalatal and retroglossal regions contribute to the specific morphology of the airway of a given individual.32 Collapse of the upper airway reduces its airway intraluminal diameter and increases airway resistance, leading to the apneas and hypopneas that characterize OSA.33,34 Figure 99-1 displays a midsagittal magnetic resonance image in a normal individual in which the retropalatal and retroglossal regions are outlined. This midsagittal image also highlights the airway, tongue, soft palate, mandible, and subcutaneous fat. The lateral upper airway soft tissue structures of the retropalatal region, that is, the lateral pharyngeal walls and lateral parapharyngeal fat pads, are depicted in Figure 99-2.
Figure 99-2 Axial MR image at the retropalatal level in a normal subject. The relevant soft tissue and bony structures surrounding the upper airway are highlighted. The tissues immediately lateral to the airway are the lateral pharyngeal walls and the parapharyngeal fat pads. (Reproduced with permission from Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1): 1673–1689.)
Anatomical factors are clearly a major contributor to the propensity for airway collapse during sleep. In addition, the critical role of neuromechanical control of the upper airway in the pathophysiology of OSA is becoming increasingly well understood.
 ANATOMIC FEATURES THAT PREDISPOSE TO OBSTRUCTIVE SLEEP APNEA
ANATOMIC FEATURES THAT PREDISPOSE TO OBSTRUCTIVE SLEEP APNEA
The extrathoracic upper airway lacks a robust support framework of cartilaginous rings and therefore is at risk for collapse due to extraluminal tissue pressure exerted by circumferential craniofacial and soft tissue structures and negative pressure associated with inspiration. Changes in pharyngeal transmural pressure, defined as the difference between the pressure in the airway lumen and the pressure exerted by tissues surrounding the site of collapse, modulate upper airway size. Activity of the pharyngeal dilator muscles offsets extraluminal tissue pressure to help maintain upper airway patency.35
Among individuals with sleep apnea, upper airway collapse occurs most commonly in the retropalatal and retroglossal regions, reducing airway intraluminal diameter and increasing airway resistance, which leads to apneas and hypopneas.36 Multiple potential sites of upper airway collapse have been visualized in many patients with OSA and tend to be reproducible from episode to episode.37 During wakefulness, upper airway caliber is smaller in patients with sleep apnea compared to normal individuals.38 Habitual snorers also have generalized narrowing of the pharyngeal lumen compared to normal subjects, regardless of whether they are obese.32 Nevertheless, smaller upper airway caliber does not entirely explain OSA risk: Men have larger pharyngeal airway cross-sectional area and airway volumes than women, but are more likely to develop sleep apnea. Recently, attention has focused on the role of airway length to explain gender disparity in OSA risk, given observations that men have longer, and thus more collapsible, pharyngeal airways compared to women.39 A reduction in the lateral diameter of the airway in individuals with OSA, with relative preservation of the anterior–posterior diameter, may also promote upper airway dysfunction.40
In population studies, neck circumference is a strong predictor of OSA.41 Consistent with this observation, individuals with OSA demonstrate an excess of upper airway soft tissue for the space within the craniofacial structures that envelop the pharyngeal lumen. Patients with sleep apnea have larger tongues and longer soft palates than normal subjects (Fig. 99-3).39,42–44 The larger the volume of the lateral pharyngeal walls, tongue, and total upper airway soft tissue (Fig. 99-4), the greater the likelihood of having OSA; specifically, increased thickness of the lateral pharyngeal walls may contribute to narrowing of the apneic airway.42 Imaging studies have demonstrated that the total volume of fat surrounding the airway is also greater in apneic individuals compared to BMI-matched normal subjects.45 These findings suggest that fat deposition in the neck—in the parapharyngeal fat pads, under the mandible, and particularly in the tongue—may similarly reduce upper airway caliber. Increased soft tissue mass may increase tissue pressure, resulting in airway collapse and decreased airway volume.35
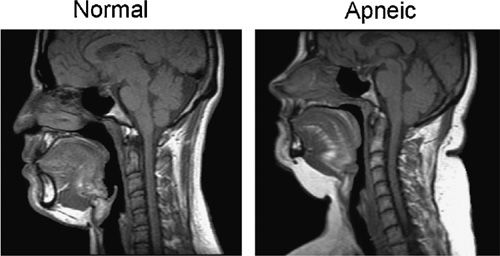
Figure 99-3 Midsagittal magnetic resonance imaging (MRI) of a normal subject on the left and a patient with sleep apnea on the right. The upper airway is smaller in both the retropalatal and retroglossal region in the apneic patient. The soft palate is longer in the apneic patient. The tongue is bigger in the retroglossal region in the patient with sleep apnea. The amount of subcutaneous fat (white area at the back of the neck) is greater in the apneic. (Reproduced with permission from Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–1689.)
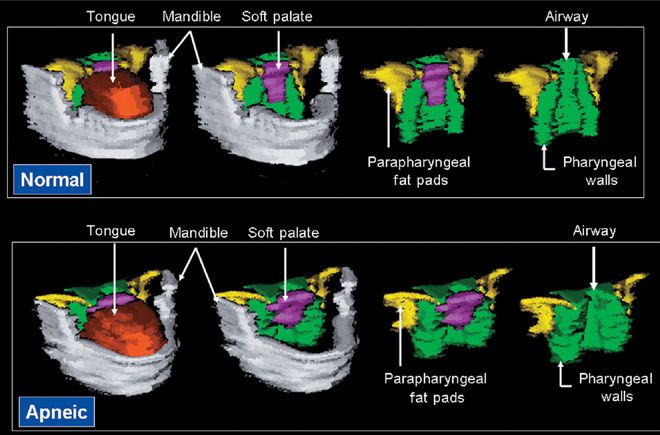
Figure 99-4 Volumetric reconstruction of axial magnetic resonance (MR) images in a normal subject (top panel) and a patient with sleep apnea (bottom panel). The mandible is depicted in gray, the tongue in orange/rust, the soft palate in purple, the lateral parapharyngeal fat pads in yellow, and the lateral/posterior pharyngeal walls in green. Both subjects had an equivalent body mass index (32.5 kg/m2). Upper airway caliber is greater in the normal subject than in the patient with sleep apnea. The tongue, soft palate, and lateral pharyngeal walls are all larger in the patient with sleep apnea than in the normal subject. (Reproduced with permission from Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530.)
Variations in craniofacial morphology also influence upper airway configuration. For example, retroposed mandible and increased hyoid–mandibular plane distance have been associated with higher risks of apnea.46–48
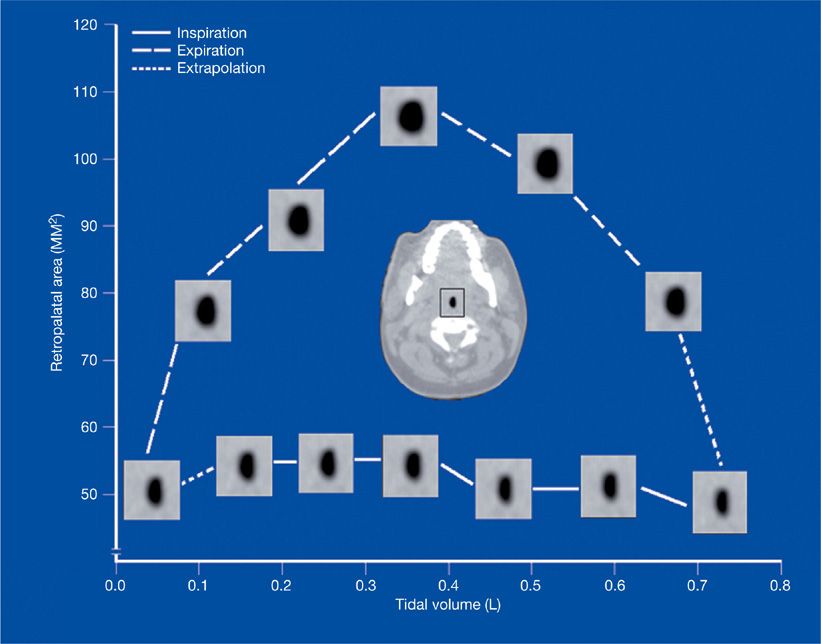
Changes in upper airway anatomy during the respiratory cycle can influence the propensity for airway collapse during both inspiration and expiration. Using dynamic upper airway imaging with CT, MRI, and nasopharyngoscopy, the airway’s geometrical changes have been partitioned into three phases (Fig. 99-5). During inspiration, upper airway area is relatively constant, implying a balance between muscle dilator activity and negative airway lumen pressure. In early expiration, the activity of airway dilator muscles decreases, intraluminal pressure rises, and the airway widens maximally. At end expiration, upper airway dimensions decrease. Patients with OSA have more narrowing during end expiration compared to normal controls.40,49,50
Figure 99-5 Changes in upper airway area as a function of tidal volume during the respiratory cycle using cine CT (computed tomography). Airway caliber is relatively constant in inspiration. Airway size increases in early expiration and decreases in late expiration. (Reproduced with permission from Schwab RJ, Gefter WB, Hoffman EA, et al. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep-disordered breathing. Am Rev Respir Dis. 1993;148(5):1385–1400.)
A number of recent studies have also demonstrated that redistribution of extracellular fluid can occur during sleep and in the supine position, leading to significant increases in neck circumference and airway collapsibility, and a reduction in upper airway cross-sectional area.51–53 The magnitude of fluid redistribution is associated with the degree of SDB, suggesting a contributory role for this mechanism in the development of OSA. However, absolute AHI fell only modestly in a small crossover study in which patients wore compression stockings during the daytime to reduce nocturnal fluid shifts, suggesting that this mechanism does not play a major role in OSA pathogenesis.54
 NEURAL MODULATION OF UPPER AIRWAY PATENCY
NEURAL MODULATION OF UPPER AIRWAY PATENCY
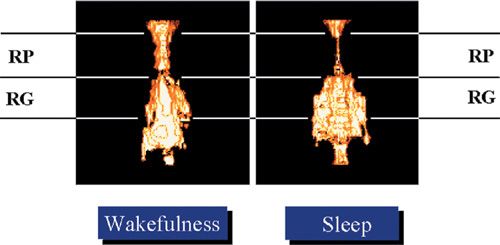
During sleep, the balance in transpharyngeal pressure shifts toward upper airway collapse due to reduced upper airway dilator muscle activity. MR images suggest that even among normal individuals, the retropalatal upper airway narrows during sleep (Fig. 99-6). The neural control of the upper airway muscles is complex and involves several neurotransmitters (serotonin, norepinephrine, orexin–acetylcholine, and gamma-aminobutyric acid) that are influenced by sleep itself.55–57 The most widely studied upper airway muscle is the genioglossus.
Figure 99-6 Volumetric state–dependent airway imaging in a normal subject using magnetic resonance imaging (MRI). Airway volume during sleep is smaller in the retropalatal (RP) region but not the retroglossal (RG) region. Such images suggest that the upper airway during sleep does not narrow as a homogeneous tube. Nonetheless such images indicate that the upper airway of subjects without sleep apnea narrows during sleep. (Reproduced with permission from Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530.)
Three neural mechanisms have been shown to be operative with regard to genioglossus muscle activity and vulnerability to obstructive apneic events. First, negative airway pressure detected by laryngeal mechanoreceptors activates the genioglossus via increased hypoglossal nerve discharge. This reflex is diminished during non-REM sleep compared to wakefulness, and is further reduced during REM sleep, placing the airway at risk for collapse.58–61 Recently, a novel, state-sensitive motor inhibitory cholinergic channel that operates at the hypoglossal motor pool has been identified as the principal mechanism of REM sleep pharyngeal motor inhibition.62 Furthermore, patients with OSA have reduced upper airway reflexes during sleep compared to normal individuals.63 Whether these differences occur due to individual variability in neuromuscular responses placing some individuals at increased risk for OSA, or whether repetitive injury from snoring vibration or oxidative stress leads to neural or muscular damage have been debated.64–66
Second, the upper airway muscles respond to input from the respiratory control center in the medulla, with increases and decreases in activity in proportion to respiratory drive. In situations of ventilatory control instability in which respiratory drive waxes and wanes, diminished upper airway muscle activity can precipitate airway collapse, that is, obstructive apnea.67,68 Third, neural mechanisms modulating arousal (serotonergic and noradrenergic neurons) have a tonic excitatory influence on genioglossus activity. With the onset of sleep, there is a reduction in arousal-modulated excitatory output to the upper airway musculature.69,70 During episodes of upper airway collapse, arousal from sleep in response to respiratory activation helps to restore airway patency.71,72 Although arousals are not always necessary for recovery from upper airway obstruction, patients with OSA have a diminished ability to restore ventilation without cortical arousal compared to nonsnorers.73
 THE APNEIC EVENT
THE APNEIC EVENT
Occlusion of the airway results in a range of immediate physiologic disturbances. Episodic reductions or cessations in ventilation despite continued respiratory efforts, recurrent episodes of intermittent hypoxemia, and frequent arousals from sleep form the basis for a cascade of downstream perturbations. During obstructive apneas, breathing efforts create abrupt reductions in intrathoracic pressure that can increase atrial natriuretic peptide (ANP) release and left ventricular (LV) transmural pressure, compromise LV filling and increase both preload and afterload.74–78 Myocardial oxygen demand increases despite reduced coronary blood flow and decreased oxygen delivery due to apnea-related hypoxia.79 Surges in sympathetic nervous system activity that occur due to apnea, hypoxia, hypercapnia, and arousal result in increased peripheral resistance and cardiac stimulation, which in turn lead to postapneic increases in blood pressure (BP) and heart rate.80–83 These elevations in sympathetic activity persist during the day in individuals with OSA.84 Intermittent hypoxemia is associated with increased production of reactive oxygen species and oxidative stress, which can impair endothelially mediated vasodilation, contributing to BP elevation.85,86 Reactive oxygen species also increase activity of the transcription factor nuclear factor kappa B (NF-κβ), a key component of the inflammatory response.87 NF-κβ stimulates the production of inflammatory mediators (e.g., C-reactive protein, tumor necrosis factor-α, and interleukins 6 and 8) and adhesion molecules (platelet endothelial adhesion molecule, intercellular adhesion molecules [ICAMs]).88–92 These effects of intermittent hypoxia are likely to promote endothelial damage and atherosclerosis.93,94
Termination of obstructive apneas usually occurs with a transient arousal or awakening from sleep. Arousals are coupled with respiratory effort and involve both chemical (hypoxia) and mechanical (increased respiratory effort against an occluded airway) stimuli.95,96 Arousal mechanisms can be adversely affected by alterations in chemosensitive systems, ingestion of alcohol, or use of sedatives and hypnotics, leading to prolongation of apneic events.
EPIDEMIOLOGY AND RISK FACTORS FOR OSA
The section focuses on the epidemiology of obstructive sleep apnea and the many risk factor for the disorder.
 EPIDEMIOLOGY OF OSA
EPIDEMIOLOGY OF OSA
Estimates of the prevalence of OSA in the general population are variable and dependent on several factors, including the characteristics of the population studied (e.g., obesity, ethnicity), methods used to measure SDB, disease definitions (e.g., hypopnea definition), and the AHI threshold used to define OSA.17,97,98 In the United States, a landmark community-based population study (the Wisconsin Sleep Cohort Study) published in 1993 reported the prevalence of OSA with daytime sleepiness (i.e., OSAS) to be 4% in men and 2% in women between the ages of 30 and 60.99 Subsequently, additional epidemiologic studies from a diverse array of countries including Australia, the United States, China, India, Korea, Spain, and Brazil have estimated the prevalence of symptomatic OSA to be between 3% and 8% in men and 1% and 5% in women.17,99–107 In fact, current prevalence is likely to be substantially higher, given rising rates of obesity and the aging of the population worldwide.108
The prevalence of OSA defined by the presence of SDB events alone, without daytime symptoms, is also considerably higher than the prevalence of symptomatic OSA.17,99,106 For instance, 24% of men and 9% of women in the Wisconsin Sleep Cohort Study had an AHI of at least 5 events/h.99 However, the impact of milder, asymptomatic OSA on adverse health outcomes remains an area of active debate.
Age
The effect of age on the prevalence of OSA is complex. Population studies have demonstrated that the prevalence of OSA increases through midlife, but then plateaus after age 60 to 65.109 Furthermore, after controlling for obesity, mean OSA severity may decline with age.17 Nevertheless, individuals between ages 65 and 100 are more than six times more likely to have OSA compared to men and women 20 to 44 years of age.17,101 Several studies have observed that the prevalence of SDB is greater than 50% in those at least 65 years of age.97,106,109,110 These findings have raised questions about whether there is a “survivor effect” for OSA among older adults (i.e., whereby those with OSA who survive to old age are relatively resistant to its adverse effects). Thus, the clinical significance of OSA in the elderly is debated, as is whether a higher threshold should be used to define OSA in this age group.111
Gender
Epidemiologic studies demonstrate that the prevalence of OSA is two to three times higher in men than women and that postmenopausal women are at an approximately threefold higher risk for OSA compared to premenopausal women.17,99,105,112 The lower risk among premenopausal women appears to be related to the effects of reproductive hormones; however, data describing how hormone levels affect upper airway anatomy and function or the control of breathing leading to SDB remain limited.113–115
Gender differences in the prevalence of OSA may also be related to differences in body fat distribution. Men exhibit a more central fat distribution, including the neck, thereby increasing the risk for narrowing and closure of the upper airway.116–118 Among women, pregnancy poses an additional period of vulnerability for SDB, with important potential consequences for maternal and fetal well-being.119,120 The prevalence of self-reported snoring increases with advancing pregnancy and has been estimated to be between 14% and 46%.121,122 Further work is needed to define the incidence, prevalence, and impact of gestational sleep apnea.
Ethnicity
While the majority of population-based prevalence estimates for OSA are derived from Caucasian populations, several recent studies have sought to characterize the burden of OSA in several Asian countries, in South America (Brazil), and among African-Americans and Hispanics in the United States.102–105,107 In Hong Kong, India, and Korea, the prevalence of OSA is similar to Caucasian population samples, despite the lower prevalence of obesity among Asians. In fact, after controlling the effects of age and obesity, OSA severity appears to be higher among Asians, perhaps due in part to craniofacial characteristics.123–126
Among middle-aged African-Americans, the prevalence of OSA is comparable to Caucasians.109,127 A higher prevalence of OSA has been observed, however, among young African-Americans (<25 years of age) compared to either Caucasians or African-Americans in other age groups.127,128 Among Hispanics in the United States, snoring is more common than among Caucasians.129,130 Emerging data suggest that the prevalence of OSA may be higher among Hispanics compared to non-Hispanic whites.131–133 Data on OSA prevalence in other ethnic groups and countries are relatively sparse at present.
 RISK FACTORS FOR SLEEP APNEA
RISK FACTORS FOR SLEEP APNEA
Risk factors for OSA are summarized in Table 99-1.
Obesity
After rising dramatically during the last decades of the 20th century, the rate at which obesity is increasing in the United States appears to be slowing or reaching a plateau.134–137 Worldwide, the prevalence of obesity has nearly doubled since 1980.138 The increase in obesity prevalence is important because numerous epidemiologic studies have shown that obesity is the strongest risk factor for OSA in adults, and that a dose–response relationship exists between excess body weight and sleep apnea prevalence, such that the prevalence of OSA is greatest among individuals in the highest BMI categories.17,100–106 Although the majority of these investigations were cross-sectional, several longitudinal studies have demonstrated that weight gain accelerates disease progression. Interestingly, among the elderly, the influence of obesity is diminished compared to its effect in younger adults,109 which may be due to accelerated mortality among obese individuals with OSA (i.e., survivorship bias) and the greater role of other age-related risk factors for OSA.
Individuals with OSA have been observed to have larger neck circumferences than those without.41 Imaging studies have demonstrated that obese individuals with OSA have larger soft tissue structures compared to normal individuals, and BMI has been shown to correlate with the percentage of fat deposition within the tongue.42,139 As noted earlier, the larger size of the soft tissue structures may promote pharyngeal collapse during sleep by increasing tissue pressure on the pharyngeal wall.43,45,140 In addition to biomechanical effects associated with larger soft tissue structures, obesity is also likely to have important interactions with other risk factors, such as craniofacial characteristics.
Anatomical factors alone, however, do not appear to fully explain the relationship between obesity and the development of OSA. Recent experiments have demonstrated that mice deficient in leptin, a hormone produced by adipocyte tissue that plays a major role in regulating appetite and metabolism, have more collapsible airways independent of weight.141 Furthermore, leptin replacement can mitigate upper airway obstruction through a combination of peripheral mechanical and central neuromuscular actions. Obese humans, however, are generally leptin resistant and have high circulating levels of leptin, rather than leptin deficiency.142 Other pathways via which obesity may increase the propensity for OSA include effects on ventilatory control mechanisms; neural compensatory mechanisms that maintain airway patency; and changes in lung volumes. Nonanatomic phenotypic traits such as genioglossus muscle responsiveness, arousal threshold, respiratory control stability, and loop gain appear to be particularly important in determining whether individuals with less collapsible airways manifest OSA.143
Genetic Factors
Evidence that genetic factors are involved in the pathogenesis of sleep apnea continues to accumulate. A number of studies initially demonstrated the tendency for OSA to aggregate in families.144,145 More recently, studies utilizing evolving genetic epidemiology techniques have demonstrated increased risk for OSA, independent of obesity, among first-degree relatives in geographically diverse populations in Iceland, Israel, Scotland, and Cleveland (USA).46,146–148 A recent review documented 50 associations between genetic polymorphisms and OSA that have been identified in over 31 published candidate gene studies (for details, see Varvarigou et al, SLEEP 2011).149 The majority of these candidate gene studies had inadequate sample sizes. Among these, four of the polymorphisms were observed in at least three separate studies. However, only one gene variant (TNFA rs1800629) remained significantly associated with OSA using meta-analysis techniques.149 A genome-wide association study in OSA has not yet been published. Ultimately, given the complexity of SDB, many different genes are likely to contribute.
Craniofacial Anatomy
A proportion of patients with OSA are not obese. In nonobese individuals, craniofacial features such as retroposed mandible, micrognathia, and narrowing of the hard palate are the primary risk factors for apnea.46–48 Furthermore, heritability of craniofacial abnormalities (retroposed mandible, inferior displaced hyoid bone) and upper airway soft tissue structures (tongue volume, lateral pharyngeal walls, and total soft tissue) has been demonstrated in first-degree relatives and siblings.46,150,151 These factors may operate in concert or alone to increase the risk of apnea. Genetic disorders associated with craniofacial and/or upper airway soft tissue abnormalities, including Treacher Collins syndrome, Down syndrome, Apert syndrome, and Pierre Robin syndrome, also confer increased risk of SDB.152
Endocrine Abnormalities
Individuals with specific endocrine conditions are at risk for OSA. Hypothyroidism, especially when accompanied by myxedema, is associated with both obstructive and CSA due to alterations in muscle function and blunted ventilatory response.153,154 The presence of a goiter may place the patient at risk for OSA155; and thyroid surgery (lobectomy or total thyroidectomy) can improve OSA symptoms including snoring and excessive somnolence.156 Macroglossia associated with hypothyroidism also contributes to the development of OSA. OSA is common and often severe in patients with acromegaly, presumably related to both osseous and soft tissue changes narrowing the upper airway.157
Given the low prevalence of OSA among premenopausal women compared to postmenopausal women and men, the female reproductive hormones appear to have a protective effect on OSA risk. Interestingly, among women with polycystic ovarian syndrome, which is characterized by hyperandrogenism, obesity, and cardiometabolic dysfunction, OSA risk is markedly higher compared to weight-matched controls.158,159 OSA risk among postmenopausal users of hormone replacement therapy is similar to that of premenopausal women and lower compared to nonhormone users.17,112 However, due to increased risk of cardiovascular disease and breast cancer and equivocal data from small treatment trials, hormone replacement therapy is not recommended as a treatment for OSA.160–164
Other Considerations
Alcohol, which reduces upper airway tone, and sedatives or hypnotics, which reduce the arousal mechanism, can also worsen OSA.165,166 Each potential risk factor should be considered in the patient evaluation, as it is important to address why an individual has developed sleep apnea.
CLINICAL PRESENTATION AND SCREENING FOR OSA
Elements in the history and physical examination, as well as screening tools useful in establishing a diagnosis of OSA, are discussed below.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
History
The diagnosis of OSA is frequently suggested by the history. Patients with sleep apnea often complain of both daytime and nighttime symptoms (Table 99-2). Bed partners are crucial informants of nocturnal events, and when available, a detailed history from the bed partner is imperative in cases of suspected OSA. Snoring is the cardinal complaint reported by the bed partner. Typically, the snoring is loud, nightly, and has existed for many years. Snoring may be so disruptive that the bed partner can be driven to sleep in another room.167 A bed partner may report a witnessed apnea that is followed by a loud snort or gasp at the end of the apneic episode. This can be extremely concerning to the partner and serve as the trigger for seeking medical attention.
Individuals with OSA may also report snorting or gasping, choking, nocturnal diaphoresis, and restlessness related to airway obstruction.168,169 A sensation of choking or dyspnea is reported in up to 30% of patients. Frequent nocturnal awakenings related to repetitive airway obstruction lead to sleep fragmentation. Nocturia is fairly common and has been attributed to ANP release in response to apnea-related right atrial stretch.78 However, while a large community-based epidemiologic study recently found a dose–response relationship between the severity of sleep apnea and levels of brain natriuretic peptide (BNP), this relationship was not observed in a similar analysis performed in a subset of participants from the Sleep Heart Health Study.170,171
Repetitive apneic events are not conducive to restorative sleep. Some studies have shown that untreated OSA patients have decreased slow wave sleep (non-REM stage 3) and REM sleep and more sleep stage transitions.172,173 Thus, individuals with OSA are frequently unrefreshed upon awakening in the morning. Morning headaches are a less common manifestation of sleep apnea. When reported, the possibility of hypercapnia due to OHS should be considered.174
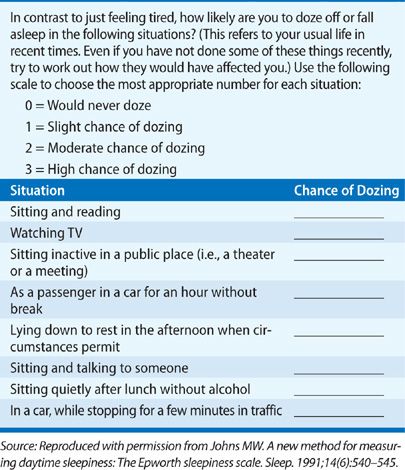
Excessive daytime sleepiness is a chief clinical consequence among patients with OSA.175 Less commonly, patients may report insomnia, with difficulties initiating and maintaining sleep.176,177 Interestingly, although the proportion of individuals who endorse daytime sleepiness increases with OSA severity, disease severity has proven to correlate only modestly with the degree of excessive somnolence.178,179 Typically, the excessive daytime sleepiness of individuals with OSA occurs in sedentary situations, for example, following meals, watching television, reading, and during conversation. Driving is particularly problematic in patients with sleep apnea.180,181 Patients may report falling asleep at red lights or stop signs, drowsiness while driving, and in extreme cases, motor vehicle accidents.182 Thus, it is imperative to inquire about drowsy driving, which is risky not only to the patient but also to others on the road. The Epworth Sleepiness Scale (Table 99-3), a questionnaire for assessing the degree of subjective sleepiness, is usually elevated in sleep apnea patients (scored 0–24, with >10 considered abnormal) and can be a useful tool for assessing sleepiness in clinical settings.178 Nevertheless, it is important to appreciate that the presence of excessive daytime somnolence is neither a necessary nor a sufficient condition to make the diagnosis of OSA: Many individuals with an elevated AHI do not report daytime sleepiness and others report sleepiness in the absence of sleep apnea (often due to insufficient sleep).
OSA can also cause symptoms related to cognitive impairment.183–186 Inattention and deficits in memory and concentration often affect the ability to function at work. Fear of falling asleep inappropriately may limit an individual’s willingness to integrate socially.187 Moreover, patients with sleep apnea and/or their spouses can report irritability, depressive symptoms, and personality change.188,189 Sexual dysfunction, that is, either decreased libido or impotence, is another common complaint among men with OSA.190
Physical Examination
The examination of the patient with suspected OSAS should focus on careful inspection of the head and neck, determination of the presence of obesity (BMI > 30 kg/m2), and measurement of BP. Neck circumference greater than 17 in (43.2 cm) in men or 16 in (40.6 cm) in women suggests an increased risk for OSA.191 The evaluation should also include assessment for abnormalities of the shape and size of the craniofacial structures (e.g., mandibular retrognathia, micrognathia, crossbite, narrowing of the hard palate, and dental malocclusion); evaluation for factors contributing to nasal obstruction including nasal asymmetry, polyps, septal deviation, and hypertrophy of the turbinates; and visualization of the pharynx to assess crowding and soft tissue dimensions (macroglossia, tonsillar hypertrophy, lateral peritonsillar wall narrowing, enlarged/elongated uvula, or a narrow, high-arched hard palate). The modified Mallampati classification is often used to describe the patency of the upper airway, with increased risk for sleep apnea observed among individuals with class III or IV airways.192
 SCREENING FOR SLEEP APNEA
SCREENING FOR SLEEP APNEA
Inexpensive tools have been developed to assess the likelihood of apnea. The ability of a number of standardized instruments, such as the Multivariable Apnea Prediction (MAP) Index and the Berlin questionnaire, to identify individuals at risk for sleep apnea have been evaluated.168,193 The sensitivity and specificity of these questionnaires tend to vary depending on the population studied, the specific cutoffs chosen and the severity of OSA to be identified.194 Recently, in the surgical preoperative setting, an 8-item questionnaire (STOP-BANG) has been shown to have good predictive value for identifying severe OSA.195 Other clinical situations in which sleep apnea should be considered in evaluating patients are outlined in Table 99-4.