SINGLE VENTRICLE PHYSIOLOGY
Introduction
The normal heart is a 2-pump organ, with one pumping exclusively to the body and the other pumping exclusively to the lungs. This chapter deals with structural defects that result in only one functional ventricle. In most cases, the defect involves the underdevelopment of one of the pumping chambers. Occasionally, there may be 2 ventricles that have inlets or outflow tracts that cannot be separated. Some diagnoses, for example, tricuspid atresia, are always a functional single ventricle, while others, such as pulmonary atresia with intact ventricular septum (PA-IVS) are often, but not always, single-ventricle lesions. Other lesions (eg, Ebstein’s anomaly) are usually handled as biventricular repairs, but occasionally may need to be handled as single ventricle lesions. In fact, determining whether to treat a complex lesion as one- or two-ventricle repair can be one of the most challenging issues for cardiologists and surgeons. Specific algorithms to determine whether lesions should be on a single ventricle pathway or a biventricular pathway are beyond the scope of this chapter. This chapter also does not deal with 2 ventricle hearts which are palliated with single ventricle-like circulations until patients can undergo biventricular repairs.
This chapter will describe the broad principles of single ventricle physiology (SVP) and the overall strategies for surgical palliation. Some general outcome data will also be presented. Detailed discussions of specific lesions are beyond the scope of this chapter (see Chapters 30, 33, and 37 for further discussion).
For the purpose of this chapter, SVP with pulmonary blood flow (PBF) supplied by a ventricular or great vessel level connection is referred to as the “systemic–pulmonary connection” or “Stage I.” SVP with PBF supplied by cavopulmonary connection is referred to as “Stage II.”
Stage I: Systemic–pulmonary Connection
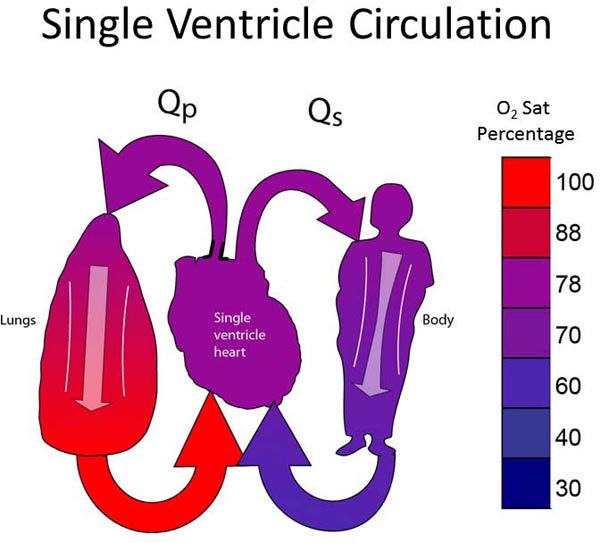
All neonates with SVP have Stage I physiology (Figure 18.1). The single ventricle must supply blood to both pulmonary and systemic circulations. The ventricle pumps, and at some point there is a “fork in the road,” where some of the blood continues to the body and some is diverted to the lungs. PBF may arise from a pulmonary artery (PA) coming from the single ventricle; that is, the single ventricle gives off both an aorta and a PA (as depicted in Figure 18.1) or, PBF may derive from the level of the great vessels (patent ductus arteriosus [PDA], surgically placed systemic–PA shunt, or aortopulmonary collateral vessels). There are some physiological differences between PBF arising from the ventricle and PBF arising from the great vessels, though a detailed discussion of the differences is beyond the scope of this chapter.
Figure 18.1. The ideal single-ventricle circulation has balanced Qp:Qs, pulmonary venous return fully saturated, and a systemic arterial-venous extraction of 20% to 25%.
Before describing the ideal Stage I circulation, some underlying principles need to be clarified. These “rules of SVP” are used to derive logical management strategies:
- Pulmonary vascular resistance (PVR) is less than systemic vascular resistance (SVR).1,2
- Although this statement is certainly true in general, this principle is not static. PVR is relatively high in the immediate postnatal period and falls afterward over hours, days, and weeks.3 Even though PVR<SVR, the exact ratio between them may vary, and the PVR:SVR ratio can fluctuate over time.
- The ideal ratio of pulmonary to systemic blood flow (Qp:Qs) is roughly 1:1.4,5
- Although it can be argued that the ideal Qp:Qs is not exactly 1:1, there is a consensus that ideal Qp:Qs is relatively close to 1:1. Qp:Qs less than 1 results in excessive cyanosis. Qp:Qs greater than 1 results in decreased systemic perfusion and/or an excessive volume load on the ventricle.
- The pulmonary outflow tract must have moderate stenosis.
- Combining the first 2 rules, the ideal circulation must have some pulmonary outflow stenosis. If PVR<SVR and there is no obstruction to PBF, PBF will be excessive. Moderate constriction of the pulmonary outflow (or PAs) is necessary to keep Qp:Qs balanced.6
- The systemic circulation should never be obstructed.
- Systemic outflow tract obstruction is simply an unnecessary afterload on the single ventricle. Since Stage I physiology already has an inherent volume load on the ventricle, an additional afterload is poorly tolerated.6,7
- Pulmonary venous blood should be fully saturated.
- There is no benefit to pulmonary venous desaturation. Although it is accepted that systemic arterial saturations (SaO2) should not be too high8,11,12 (described further below), this should not be confused with pulmonary venous desaturation. While it is detrimental for too much blood flow to go to the lungs, all of the blood that does go through the lungs should be fully saturated.
- Normal systemic arterial minus venous oxygen saturation is 20% to 25%.9–12
- The body will extract a typical amount of the oxygen content of the systemic blood flow (SBF) if cardiac output and hemoglobin levels are normal. Thus, if arterial saturation is 100%, systemic venous saturation will be 75% to 80%. If arterial saturation is 80%, systemic venous saturation will be 55% to 60%.
- Systemic and pulmonary outflow tracts are never both (naturally) obstructed.
- This is an observational fact. Very likely, systemic and pulmonary outflow obstruction does occur in the same fetus, though the fetuses do not survive to live birth. Of babies born at a viable gestational age, it is virtually never observed to have both outflow tracts obstructed. One of the outflow tracts may be obstructed, or both may be unobstructed. The corollary is that if one outflow is known to be obstructed (or atretic), the other must be unobstructed.
The Ideal Stage I Circulation
The ideal Stage I circulation is depicted in Figure 18.1. The single ventricle gives off SBF which is unobstructed. There is a pulmonary outflow tract with moderate stenosis which allows an adequate, but not excessive amount of PBF. As described in the “Rules of SVP” above, the ideal single ventricle circulation assumes a pulmonary venous saturation of 100%, Qp:Qs of 1:1, and a systemic arterial–venous oxygen extraction (ΔA−V O2) of 25%. The resulting arterial oxygen saturation will be 75% and the venous saturation will be 50%. If ΔA−V O2 is 20%, arterial oxygen saturation will be 80% and venous saturation 60%. This is how the “ideal” oxygen of 75%–80% is derived for patients with SVP.9,10
Surgical Options for Neonates with SVP
Given the above principles of SVP, there are 4 broad surgical treatment groups which can be applied to all patients with SVP. This section deals with structural variations in SVP (eg, obstructed pulmonary outflow tract) as opposed to functional variation (eg, poor cardiac contractility). The groups* are as follows:
- Group 1: Unobstructed SBF
- 1A: Unobstructed SBF with unobstructed PBF
- 1B: Unobstructed SBF with moderately obstructed PBF
- 1C: Unobstructed SBF with severely obstructed (or atretic) PBF
- 1A: Unobstructed SBF with unobstructed PBF
- Group 2: Obstructed SBF
*Note: The classification described above was created for this chapter. It is not a generally accepted classification scheme for neonates with SVP.
Because the ideal single-ventricle circulation invokes unobstructed SBF and moderately obstructed PBF, patients with unobstructed blood flow to the body may either have unobstructed PBF (excessive PBF), severely obstructed PBF (insufficient PBF), or moderately obstructed PBF (appropriate PBF). Since any systemic obstruction is deleterious, this forms the final group. Since both outflow tracts cannot be obstructed, all cases of systemic outflow obstruction are associated with unobstructed PBF. There are no subdivisions of Group 2.
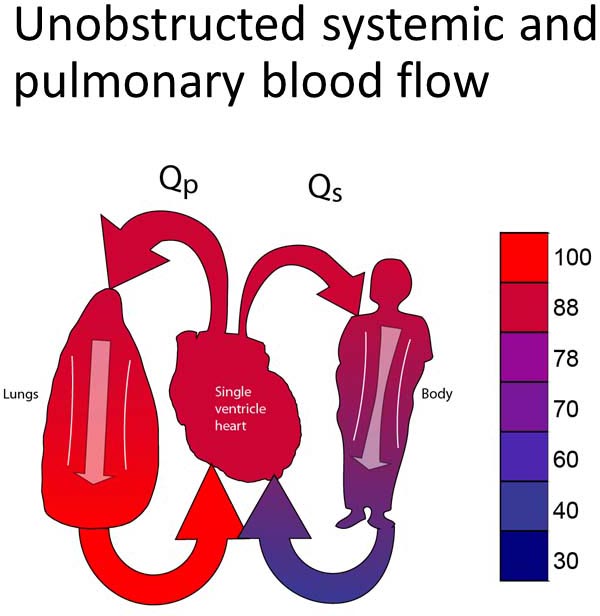
Group 1A
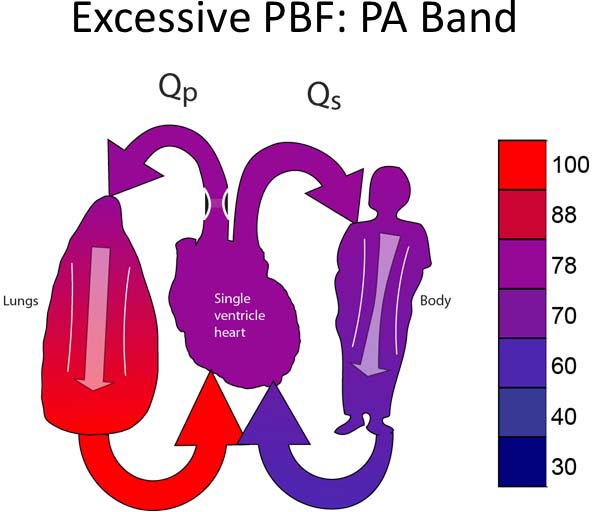
This group includes patients with unobstructed systemic and pulmonary outflow tracts (Figure 18.2A). Because of this, PBF will be excessive. The treatment for this group will be to reduce the PBF. Most typically, this will involve placing a PA band (Figure 18.2B).13–15 In some instances, it may be preferable to divide the PA and place a systemic-PA shunt to provide controlled PBF. Timing of the surgery performed is late enough to allow PVR to fall but early enough to avoid excessive heart failure symptoms or pulmonary vascular obstructive disease. Surgery would most typically be performed between 2 and 6 weeks of age.
Figure 18.2. SVP with unobstructed PBF and unobstructed SBF (A). Treatment is a PA band (B).
Group 1B
This group is born with a naturally balanced circulation; that is, unobstructed SBF and moderately obstructed PBF. These patients will not need any surgical intervention as neonates. It is worthwhile to note that balanced PBF may not stay balanced. For example, in some cases PBF is dependent on flow through a restrictive ventricular septal defect (VSD), which may become more restrictive over the first few weeks or months of life. A Group 1B lesion with appropriate PBF may thus evolve into a Group 1C lesion with insufficient PBF.16
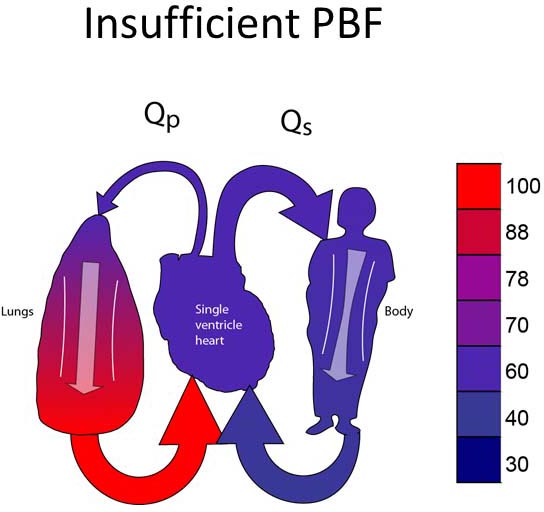
Group 1C
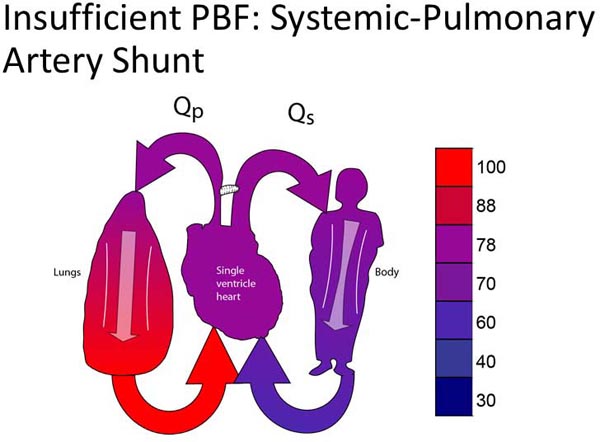
Group 1C lesions have unobstructed SBF and insufficient PBF (Figure 18.3A). Most typically, neonates in this group will have ductal-dependent PBF (pulmonary atresia or very severe pulmonary stenosis). Surgical palliation most commonly involves placement of a systemic–PA shunt (Figure 18.3B).17,18 Some patients may have PBF which is sufficient to allow the patient to tolerate ductal closure for a relatively short period of time yet still eventually require a systemic–PA shunt.
Figure 18.3. SVP with insufficient PBF (A). Treatment is placement of a systemic-PA shunt (B).
Group 2
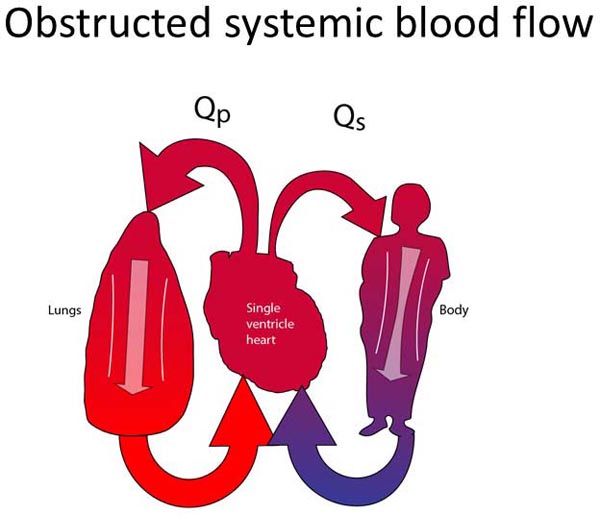
These patients have tenuous or obstructed connections between their single ventricle and the systemic circulation. In most cases, the classification of patients within this group is obvious. The left ventricle may be severely hypoplastic (assuming normally related great vessels). The aortic valve and/or the subaortic area are also commonly severely hypoplastic or atretic. Sometimes, however, it can be difficult to decide if patients belong to this group. The aortic valve and/or the subvalvar area may be marginally small. Since both outflow tracts cannot both be obstructed, PBF is always unobstructed (Figure 18.4A). The systemic outflow tract may be adequate in the neonatal period but raise concerns about still being adequate as the child grows. Sometimes, flow through the systemic outflow tract may be dependent on flow through a VSD, which is unobstructive at the time but may raise concerns about the adequacy of the VSD over the lifetime of the patient. Cardiologists and cardiac surgeons may choose to treat these neonates as Group 2 patients to prevent the future development of any obstruction to SBF.
Figure 18.4. Obstructed SBF (A) and unobstructed PBF. The surgical treatment consists of converting the pulmonary outflow into the systemic circulation. The main PA is divided and re-routed into the systemic circulation. PBF is re-established with a systemic-PA shunt (B).
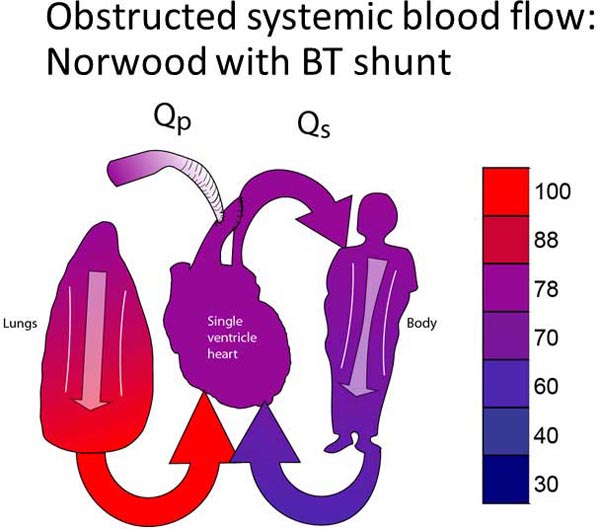
Group 2 patients are palliated with a Damus-Kaye-Stansel (DKS) procedure.19–21 A DKS procedure utilizes the concept that there is only one unobstructed outflow tract, which is currently the pulmonary outflow tract. This unobstructed outflow tract must be converted from a pulmonary outflow tract to a systemic outflow tract. Thus, the PA is divided and re-routed into the aorta. The PA branches become isolated from the heart. PBF is then re-established with a systemic–pulmonary connection (either a systemic–pulmonary shunt or a right ventricle–PA conduit). Although a DKS may be done in isolation, most patients with systemic outflow tract obstruction also have aortic arch obstruction, which also requires surgical correction. A DKS, aortic arch reconstruction, and a systemic–PA connection in combination is the more commonly performed Norwood procedure (Figure 18.4B).22–24
Role of Prostaglandins
Palliation with prostaglandins to maintain ductal patency is the norm for Group 1C patients and Group 2 patients. For patients with Group 1C lesions, ductal patency provides adequate PBF until a surgical palliation can be performed (generally a systemic–PA shunt).25 For Group 2 patients, ductal patency provides adequate SBF.26,27 As stated above, not all patients in these groups are going to be ductal dependent. It is possible for a child with borderline PBF to tolerate ductal closure with saturations, which are acceptable in the hospital but do not allow discharge. A patient with this may undergo systemic–PA shunt before discharge. Also, not all patients who are ductal dependent have single ventricle lesions (eg, tetralogy of Fallot with severe pulmonary stenosis or critical aortic stenosis). Nonetheless, the majority of single ventricle patients in Group 1C and Group 2 will require prostaglandin infusions to maintain stability prior to surgical palliation.
Other Lesions
This chapter has divided single ventricle procedures by the presence of systemic and/or pulmonary outflow tract obstruction. There are other lesions that may also need surgical management in the neonatal period. Any of the above groups may have important aortic arch obstruction, though it is most prevalent in Group 2. Systemic and pulmonary venous return must also be unobstructed. Although this may occasionally be formal venous obstruction (eg, an associated infracardiac total anomalous pulmonary venous obstruction), it more commonly is manifested in obstruction at the level of the atrial septum.28,29 In most single-ventricle lesions, either pulmonary or systemic venous flow is dependent on an adequate atrial septal defect (ASD). This must be opened in the neonatal period if it is causing systemic or pulmonary venous obstruction. Although other procedures are occasionally required, aortic arch repair and ASD enlargement are the 2 most commonly associated surgical procedures for neonates with SVP.30
Functional Variability in SVP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree