Contrast-induced nephropathy (CIN) impairs clinical outcome in patients undergoing angiographic procedures. The aim of this study was to investigate whether short-term high-dose atorvastatin load decreases the incidence of CIN after percutaneous coronary intervention (PCI). Statin-naive patients with acute coronary syndrome undergoing PCI (n = 241) randomly received atorvastatin (80 mg 12 hours before intervention with another 40-mg preprocedure dose, n = 120) or placebo (n = 121). All patients had long-term atorvastatin treatment thereafter (40 mg/day). Primary end point was incidence of CIN defined as postintervention increase in serum creatinine ≥0.5 mg/dl or >25% from baseline. Five percent of patients in the atorvastatin arm developed CIN versus 13.2% of those in the placebo arm (p = 0.046). In the atorvastatin group, postprocedure serum creatinine was significantly lower (1.06 ± 0.35 vs 1.12 ± 0.27 mg/dl in placebo, p = 0.01), creatinine clearance was decreased (80.1 ± 32.2 vs 72.0 ± 26.6 ml/min, p = 0.034), and C-reactive protein peak levels after intervention were decreased (8.4 ± 10.5 vs 13.1 ± 20.8 mg/l, p = 0.01). Multivariable analysis showed that atorvastatin pretreatment was independently associated with a decreased risk of CIN (odds ratios 0.34, 95% confidence interval 0.12 to 0.97, p = 0.043). Prevention of CIN with atorvastatin was paralleled by a shorter hospital stay (p = 0.007). In conclusion, short-term pretreatment with high-dose atorvastatin load prevents CIN and shortens hospital stay in patients with acute coronary syndrome undergoing PCI; anti-inflammatory effects may be involved in this renal protection. These results lend further support to early use of high-dose statins as adjuvant pharmacologic therapy before percutaneous coronary revascularization.
Pathogenesis of contrast-induced nephropathy (CIN) includes inflammatory mechanisms, endothelial dysfunction, and oxidative stress. Short-term statin pretreatment has improved clinical outcome through lipid-lowering independent “pleiotropic” effects in various clinical settings such as prevention of periprocedural myocardial damage during percutaneous coronary intervention (PCI) or decrease of postoperative atrial fibrillation after cardiac surgery. These are the rationales for clinical investigations concerning use of statins to prevent CIN. A previous observational nonrandomized study from our institution demonstrated that patients on long-term statin therapy at the time of PCI had lower occurrence of postprocedure CIN versus statin-naïve patients ; however, those patients received a multitude of statins at variable doses and different durations of therapy. Thus, the Atorvastatin for Reduction of MYocardial Damage during Angioplasty–Contrast-Induced Nephropathy (ARMYDA-CIN) trial was designed to test in a randomized protocol the hypothesis of whether a specific short-term pretreatment with high-dose atorvastatin decreases the incidence of CIN in patients with acute coronary syndromes (ACSs) undergoing early PCI.
Methods
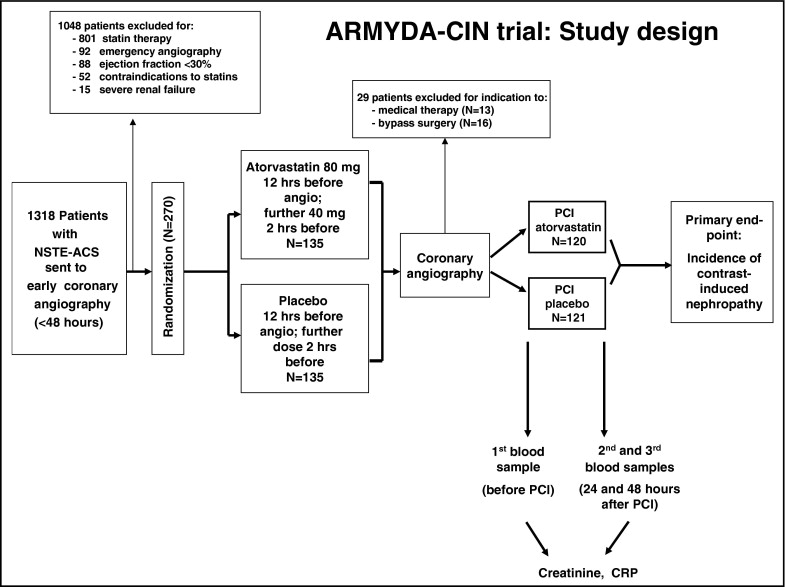
The ARMYDA-CIN trial ( Figure 1 ) is a randomized, multicenter, prospective, double-blind clinical trial performed in 2 Italian institutions (Campus Bio-Medico, University of Rome and Vito Fazzi Hospital, Lecce). Statin-naive patients with non–ST-segment elevation ACS (unstable angina or non–ST-segment elevation myocardial infarction) and a planned invasive strategy within 48 hours were included. Exclusion criteria were current or previous (<3 months) statin treatment, non–ST-segment elevation ACS with high-risk features warranting emergency coronary angiography (<2 hours), any baseline increase in liver enzymes (aspartate aminotransferases/alanine aminotransferases), left ventricular ejection fraction <30%, renal failure with a creatinine level >3 mg/dl, and a history of liver or muscle disease. In total 1,318 patients fulfilling the enrollment criteria were initially included; 1,048 patients were then excluded because of current or previous statin treatment (n = 801), indication to emergency coronary angiography (n = 92), low ejection fraction (n = 88), liver/muscle disease (n = 52), or severe renal failure (n = 15). Eligible patients were randomly allocated to receive placebo or atorvastatin (80-mg loading dose given a mean 12 hours before coronary angiography with another 40-mg dose approximately 2 hours before the procedure). Patients were assigned to the study arm using an electronic spreadsheet indicating the group assignment by random numbers; randomization blocks were created and distributed to the 2 centers. After diagnostic angiography, 29 patients who did not receive percutaneous revascularization were excluded (13 for indication to medical therapy and 16 to bypass surgery); thus, 241 patients (120 randomized to atorvastatin and 121 to placebo) undergoing PCI immediately after coronary angiography were enrolled and represent the study population. Physicians performing the procedure and follow-up assessment were not aware of the randomization assignment. Informed consent was obtained in all patients. The study was approved by the institutional review boards of the institutions involved. The trial was not supported by any external source of funding.
All interventions were performed using a standard technique. All patients received aspirin (100 mg/day) and clopidogrel 600-mg load >3 hours before the procedure. Procedural success was defined as postprocedure Thrombolysis In Myocardial Infarction grade 3 flow with decrease of stenosis to <30% residual narrowing by quantitative coronary angiographic analysis. All interventions were performed with a nonionic, low-osmolar (915 mOsm/kg), iodinated contrast agent (iobitridol, Xenetix, Guerbet, Roissy CdG Cedex, France). Glycoprotein IIb/IIIa inhibitors were administered at the physician’s discretion. During PCI, bivalirudin was used instead of unfractionated heparin in patients considered at high bleeding risk (>75 years of age, history of previous bleeding, low body weight). After the procedure, aspirin (100 mg/day) was continued indefinitely, whereas clopidogrel (75 mg/day) was continued for 1 year. All patients received atorvastatin 40 mg/day after PCI, irrespective of initial randomization assignment.
By study design blood samples were drawn before and at 24 and 48 hours after PCI for measurement of serum creatinine, with further determinations after 48 hours if clinically indicated; for this study the postprocedure peak value was used. Creatinine clearance (CrCl) was calculated by the Cockcroft-Gault formula: CrCl = ([140 − age] × weight/serum creatinine × 72) with adjustment for female gender (CrCl female = CrCl × 0.85). Patients with pre-existing renal failure (defined as preprocedural serum creatinine level ≥1.5 mg/dl or CrCl <60 ml/min) received intravenous hydration with normal saline at 1 ml/hour/kg body weight for ≥12 hours before and ≥24 hours after intervention. No specific protocol for periprocedural use of other potentially renal-protective agents was used. Maximal allowable weight- and creatinine-adjusted contrast dose was calculated by the formula: body weight (kilograms) × 5 ml/serum creatinine. C-reactive protein (CRP) levels were measured before PCI and at 24 and 48 hours after intervention by the KRIPTOR ultrasensitive immunofluorescence assay (BRAHMS, Hennigsdorf/Berlin, Germany) with a detection limit of 0.06 mg/L.
The primary end point of the trial was incidence of CIN defined as a postintervention increase in serum creatinine ≥0.5 mg/dl or >25% from baseline. The following outcomes were evaluated as secondary end points: (1) postprocedure acute renal failure defined as a rapid decrease in renal glomerular filtration with >2 mg/dl creatinine increase from baseline; (2) postprocedure levels of serum creatinine and CrCl; (3) percent variation of creatinine and CrCl after PCI versus baseline; (4) correlation of CRP peak levels after PCI with occurrence of CIN; and (5) length of stay after PCI.
If we hypothesize a 15% incidence of CIN in the control arm and a 66% decrease in the atorvastatin arm, a total sample size of 236 patients (118 in each group) would provide 80% power to detect the difference with an alpha level of 0.05. Results are expressed as mean ± SD unless otherwise specified. Continuous variables were compared by t test for normally distributed values; otherwise the Mann–Whitney U test was used. Proportions were compared by Fisher’s exact test when the expected frequency was <5; otherwise chi-square test (Yates corrected) was applied. Odds ratios and 95% confidence intervals assessing the risk of the primary end point according to potential confounding variables were assessed by logistic regression. The following parameters were evaluated first in a univariate model: study drug assignment (atorvastatin or placebo), age, gender, systemic hypertension, diabetes, left ventricular ejection fraction, previous myocardial infarction, therapy with β blockers, angiotensin-converting enzyme inhibitors and diuretics, baseline renal failure, baseline CrCl and serum creatinine, maximal contrast load, and maximal allowable contrast dose. Variables with a p value <0.15 were then entered into multivariable logistic regression analysis. All calculations were performed by SPSS 12.0 (SPSS, Inc., Chicago, Illinois) and p values <0.05 (2-tailed) were considered statistically significant.
Results
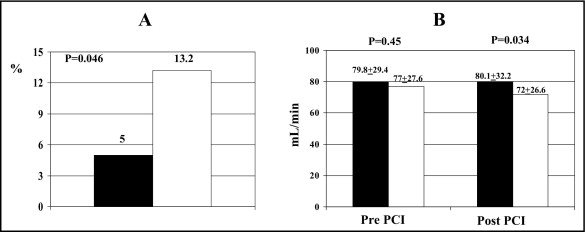
Clinical and procedural characteristics were similar in the 2 arms and are presented in Tables 1 and 2 , respectively. In particular, the proportion of diabetes mellitus was 30% in the atorvastatin and 25% in the placebo group (p = 0.64), and that of chronic renal failure was 29% and 32% (p = 0.71); baseline serum creatinine and CrCl values were 1.04 ± 0.32 versus 1.04 ± 0.22 mg/dl (p = 0.96) and 79.8 ± 29.4 versus 77.0 ± 27.6 ml/min (p = 0.45), respectively. Mean time from admission to angiography was 20 hours and procedural success was achieved in all patients; mean procedural contrast volume was not different in the 2 groups (atorvastatin 209 ± 72 ml vs placebo 213 ± 73 ml, p = 0.67), nor was the proportion of patients exceeding the maximal allowable contrast dose (17% vs 13%, p = 0.57).
| Characteristic | Atorvastatin | Placebo | p Value |
|---|---|---|---|
| (n = 120) | (n = 121) | ||
| Age (years) | 65 ± 11 | 66 ± 10 | 0.46 |
| Women | 29 (24%) | 25 (21%) | 0.62 |
| Systemic hypertension | 91 (76%) | 90 (74%) | 0.91 |
| Diabetes mellitus | 36 (30%) | 32 (25%) | 0.64 |
| Hypercholesterolemia (>200 mg/dl) | 31 (26%) | 33 (27%) | 0.92 |
| Cigarette smokers | 39 (32%) | 29 (24%) | 0.18 |
| Body mass index (kg/m 2 ) | 26.9 ± 4.3 | 26.8 ± 3.9 | 0.85 |
| Clinical presentation | |||
| Unstable angina | 77 (64%) | 82 (68%) | 0.65 |
| Non–ST-segment elevation myocardial infarction | 43 (36%) | 39 (32%) | 0.65 |
| Previous myocardial infarction | 23 (19%) | 22 (18%) | 0.97 |
| Previous coronary intervention | 11 (9%) | 12 (10%) | 0.98 |
| Previous coronary bypass surgery | 4 (3%) | 4 (3%) | 0.73 |
| Chronic renal failure | 35 (29%) | 39 (32%) | 0.71 |
| Serum creatinine (mg/dl) | 1.04 ± 0.32 | 1.04 ± 0.22 | 0.96 |
| Creatinine clearance (ml/min) | 79.8 ± 29.4 | 77.0 ± 27.6 | 0.45 |
| Hemoglobin (g/dl) | 13.7 ± 1.4 | 13.4 ± 1.7 | 0.14 |
| Left ventricular ejection fraction (%) | 54 ± 9 | 56 ± 8 | 0.07 |
| Time to angiography (hours) | 20 ± 10 | 20 ± 11 | 0.88 |
| Therapy | |||
| Aspirin | 120 (100%) | 121 (100%) | — |
| Clopidogrel | 120 (100%) | 121 (100%) | — |
| Angiotensin converting enzyme inhibitors/angiotensin-receptor antagonists | 89 (74%) | 91 (75%) | 0.97 |
| β Blockers | 43 (36%) | 36 (30%) | 0.38 |
| Calcium channel blockers | 30 (25%) | 26 (21%) | 0.62 |
| Diuretics | 15 (13%) | 12 (10%) | 0.67 |
| N -acetylcysteine | 5 (4%) | 7 (6%) | 0.78 |
| Sodium bicarbonate | 4 (3%) | 7 (6%) | 0.55 |
| Characteristic | Atorvastatin | Placebo | p Value |
|---|---|---|---|
| (n = 120) | (n = 121) | ||
| Multivessel coronary disease | 47 (39%) | 57 (47%) | 0.26 |
| Multivessel intervention | 28 (23%) | 30 (25%) | 0.91 |
| Coronary vessel treated | |||
| Left main | 1 (1%) | 1 (1%) | 0.49 |
| Left anterior descending | 69 (46%) | 66 (44%) | 0.70 |
| Left circumflex | 44 (30%) | 41 (27%) | 0.71 |
| Right | 31 (21%) | 41 (27%) | 0.26 |
| Saphenous vein grafts | 3 (2%) | 2 (1%) | 0.98 |
| Lesion B2/C | 103 (86%) | 102 (84%) | 0.88 |
| Type of procedure | |||
| Balloon only | 3 (2%) | 1 (1%) | 0.61 |
| Stent | 117 (98%) | 120 (99%) | 0.61 |
| Use of drug-eluting stents | 54 (45%) | 47 (39%) | 0.40 |
| Total stent length (mm) | 15.7 ± 4.6 | 15.1 ± 4.8 | 0.32 |
| Periprocedural antithrombotic therapy | |||
| Glycoprotein IIb/IIIa inhibitors | 27 (23%) | 23 (19%) | 0.61 |
| Unfractionated heparin | 116 (97%) | 117 (97%) | 0.73 |
| Bivalirudin | 4 (3%) | 4 (3%) | 0.73 |
| Total mean contrast volume (ml) | 209 ± 72 | 213 ± 13 | 0.67 |
| Exceeding maximal allowable contrast dose | 20 (17%) | 16 (13%) | 0.57 |
Incidence of CIN was significantly lower in patients randomized to atorvastatin (5%, 6 of 120, vs 13.2%, 16 of 121, in placebo arm, p = 0.046; Figure 2 ). Patients with CIN had received a larger contrast load versus those without CIN (240 ± 77 vs 208 ± 72 ml, p = 0.042). In the subgroup without baseline chronic renal failure, incidence of CIN was 1% in the atorvastatin versus 7% in the placebo group (odds ratio 0.15, 95% confidence interval 0.01 to 1.31, p = 0.11), whereas in patients with chronic renal failure it was 14% versus 26% (odds ratio 0.48, 0.12 to 1.80, p = 0.36).