The BEAUTIFUL Holter substudy explored the cardiac safety of the I f inhibitor ivabradine in patients with stable coronary artery disease and left ventricular systolic dysfunction receiving optimal background therapy. The Holter substudy included 840 patients (ivabradine 5 or 7.5 mg/day, n = 421; placebo, n = 419), and the safety set consisted of 807 patients (ivabradine, n = 408; placebo, n = 399). Ambulatory 24-hour electrocardiographic Holter monitoring was performed at baseline and after 1 month and 6 months. There were no relevant between-group differences in baseline characteristics; 93% were receiving concomitant β blocker. Treatment with ivabradine was associated with a decrease in 24-hour heart rate of 6.3 ± 9.5 beats/min at last assessment versus no change with placebo (0.4 ± 7.2 beats/min, p <0.001, between-group difference), with a greater decrease in waking heart rate with ivabradine than during sleep (6.8 ± 10.4 vs 5.2 ± 8.9 beats/min, respectively, at last visit). Incidence of episodes of heart rate <30 beats/min during waking hours or during sleep was ≤1% in the 2 groups. Although there were more patients with heart rates <40 or <50 beats/min with ivabradine than with placebo (awake 12% vs 4% for <40 beats/min and 68% vs 36% for <50 beats/min, respectively; asleep 22% vs 5% for <40 beats/min and 77% vs 50% for <50 beats/min, respectively), there was no between-group difference in episode severity. There was no increase in incidence of conduction and rhythm disturbances. In conclusion, our results confirm that ivabradine significantly lowers heart rate without raising concern for cardiac safety. Our observations strongly support the safety of combining ivabradine with β blockers in patients with coronary artery disease.
Ivabradine decreases heart rate by selectively inhibiting the I f current in the sinoatrial node. The I f current determines the slow diastolic depolarization of pacemaker cells—and therefore the interval between successive action potentials—making it pivotal to the control of heart rate. Moreover, the highly selective nature of I f inhibition translates into decreased heart rate without inotropic or dromotropic effects, thus preserving cardiac contractility and relaxation and atrioventricular conduction and ventricular repolarization. Randomized clinical trials have shown ivabradine to be an effective antianginal and anti-ischemic agent, alone or in combination with β blocker. Its safety has been assessed as part of a large development program including >3,500 patients with coronary artery disease (CAD) and 800 healthy volunteers. Ivabradine has been reported to have good overall cardiac safety in patients with angina, similar to that of other antianginal agents such as amlodipine and atenolol. The Morbidity–Mortality Evaluation of the I f Inhibitor Ivabradine in Patients with Coronary Disease and Left Ventricular Dysfunction (BEAUTIFUL) study provided an opportunity to test the cardiac safety of ivabradine in patients with stable CAD and left ventricular (LV) systolic dysfunction. The BEAUTIFUL population consisted of 10,917 patients recruited in 33 countries. We describe results of a preplanned Holter substudy involving 24-hour ambulatory electrocardiographic (ECG) monitoring in a subpopulation of 840 BEAUTIFUL participants.
Methods
The BEAUTIFUL trial design and procedures have been described in detail elsewhere. Briefly, this randomized double-blind placebo-controlled parallel-group trial included men and women patients with stable CAD and LV systolic dysfunction who were ≥55 years old (≥18 years if diabetic). Stable CAD was documented by previous myocardial infarction or coronary revascularization or angiographic evidence of narrowing of ≥1 coronary artery; presence of LV systolic dysfunction was defined as an LV ejection fraction <40%. Patients had to be in sinus rhythm and have a heart rate ≥60 beats/min at rest. All patients received optimal background therapy for stable CAD (β blockers, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, lipid-lowering agents, and antiplatelet agents) at stable doses for ≥1 month before entry and continuing throughout the study. After a run-in period (2 weeks) with no study treatment, patients were randomly assigned to treatment (ivabradine 5 mg 2 times/day or matched placebo). Patients with a heart rate ≥60 beats/min at rest had their treatment uptitrated at 2 weeks to ivabradine 7.5 mg 2 times/day or matched placebo. The dosage could subsequently be decreased to ivabradine 5 mg 2 times/day in patients with a heart rate <50 beats/min at rest or symptomatic bradycardia.
The Holter substudy was carried out in 102 selected centers in 16 countries with the same selection and inclusion criteria as the main study. All participants in each selected center were invited to take part in the substudy. The trial and its substudy were approved by independent ethics committees in the various countries concerned. All participants gave written informed consent before selection in the main study and signed an additional informed consent form specific to the Holter substudy.
A 24-hour digital ambulatory ECG recording was performed at baseline (between selection and randomization), at 1 month (≤5 days after uptitration), and at 6 months (or last value on treatment). Three-channel electrocardiograms were recorded on digital memory cards (SEER MC Ekpro, General Electric Marquette Medical Systems, Milwaukee, Wisconsin), which were distributed to each study center. All patients were given a diary in which they were asked to record time asleep. All memory cards were read centrally at the Montreal Heart Institute using the MARS 8000 Holter system (MARS 7.1.1, General Electric Marquette Medical Systems). ECG recordings were initially read by 1 of 3 technicians blinded to study treatment and temporal sequence of recordings and then over-read by a cardiologist.
All parameters were recorded over 24 hours and during awake and sleep periods. The main evaluation criterion was mean heart rate. Lowest heart rate was also recorded as episodes <50, <40, or <30 beats/min during awake and sleep periods. A minimum of 5 consecutive RR intervals was needed to assign a heart rate. Pauses were defined as episodes of prolonged RR interval >2, >2.5, or >3 seconds. Mechanism of the pause was also documented (sinus pause, compensatory pause after another event, pause with junctional escape, pause secondary to atrioventricular block, or pause during chronic atrial fibrillation or flutter); atrioventricular block was classified as Mobitz I or II or third-degree atrioventricular block. Other rhythm abnormalities were classified as supraventricular tachycardia, atrial fibrillation (≥30 seconds), or ventricular tachycardia. A minimum of 3 consecutive beats was required for a diagnosis of supraventricular and ventricular tachycardia. Tachycardias >30 seconds were considered sustained.
There was no sample size calculation for this exploratory study. The substudy was planned to include 700 patients (i.e., 350 per group), which was considered sufficiently large to evaluate a broad spectrum of abnormalities on Holter recordings. Descriptive statistics are provided by treatment group for baseline characteristics in the included set (i.e., all patients randomized in the main study and included in the substudy). All other results are presented for the safety set (i.e., all patients in the included set who received ≥1 dose of study treatment and had ≥1 postbaseline Holter recording of sufficient quality). Treatment effect on heart rate was analyzed in the safety set using linear models for change from baseline to last value adjusted for country, β-blocker intake at randomization, and baseline value. Cardiac abnormalities on Holter recording are presented as counts of patients in the safety set having ≥1 episode by month 1 and by last assessment (i.e., until last study drug intake plus 2 days).
Results
The BEAUTIFUL Holter substudy included 848 patients (8% of the main BEAUTIFUL population; Figure 1 ). There were no relevant between-group differences in baseline characteristics ( Table 1 ). Compared to the main BEAUTIFUL population, patients in the substudy were more likely to be in New York Heart Association class III and be receiving β blockers. The most frequently prescribed β blockers were carvedilol (mean dose 18 ± 13 mg/day), metoprolol tartrate (67 ± 40 mg/day), bisoprolol (5 ± 3 mg/day), and metoprolol succinate (59 ± 26 mg/day). Withdrawals for nonmedical reasons were more frequent than those for adverse events, and 775 patients (92%) completed the substudy ( Figure 1 ). The safety set was formed by removing 33 patients from the included set (2 patients [placebo] never received the study drug; 31 patients [ivabradine, n = 13; placebo, n = 18] had no analyzable postbaseline Holter recording) and consisted of 807 patients (ivabradine, n = 408; placebo, n = 399). Acceptable Holter recordings were collected for 96% of the population at each visit. Mean duration of Holter recording was 23.9 hours (range 19.9 to 24.1) with no difference between groups. Mean duration of awake/asleep was 15.8/8.1 hours at the 3 visits, with no difference between groups. Mean dosage of ivabradine in the substudy was 6.2 ± 1.3 mg 2 times/day at all visits. Mean treatment duration was 5.4 ± 1.7 months; 91% of the included set had compliance of 70% to 130%. No relevant between-group differences were observed.
| Variable | Holter Substudy Population | Entire Study | ||
|---|---|---|---|---|
| Ivabradine | Placebo | All | (n = 10,917) | |
| (n = 421) | (n = 419) | (n = 840) | ||
| Men | 349 (83%) | 332 (79%) | 681 (81%) | 9,047 (83%) |
| Age (years) | 64.0 ± 8.7 | 63.4 ± 8.4 | 63.7 ± 8.6 | 65.2 ± 8.5 |
| Smoker (current) | 64 (15%) | 67 (16%) | 131 (16%) | 1,647 (15%) |
| Body mass index (kg/m 2 ) | 28.7 ± 4.3 | 28.7 ± 4.2 | 28.7 ± 4.2 | 28.5 ± 4.4 |
| Previous myocardial infarction | 366 (87%) | 375 (89%) | 741 (88%) | 9,645 (88%) |
| Previous percutaneous coronary intervention | 91 (22%) | 78 (19%) | 169 (20%) | 3,286 (30%) |
| Previous bypass | 89 (21%) | 86 (21%) | 175 (21%) | 3,078 (28%) |
| Heart rate (beats/min) | 71.6 ± 10.4 | 71.5 ± 10.2 | 71.6 ± 10.3 | 71.6 ± 9.9 |
| Systolic blood pressure (mm Hg) | 128.3 ± 14.6 | 127.4 ± 14.3 | 127.9 ± 14.5 | 128.0 ± 15.6 |
| Diastolic blood pressure (mm Hg) | 78.8 ± 9.1 | 78.0 ± 8.7 | 78.4 ± 8.9 | 77.5 ± 9.3 |
| Left ventricular ejection fraction (%) | 32.7 ± 5.1 | 32.8 ± 5.0 | 32.8 ± 5.1 | 32.4 ± 5.5 |
| New York Heart Association class of heart failure | ||||
| I | 27 (6%) | 33 (8%) | 60 (7%) | 1,680 (15%) |
| II | 244 (58%) | 256 (61%) | 500 (60%) | 6,705 (61%) |
| III | 150 (36%) | 130 (31%) | 280 (33%) | 2,532 (23%) |
| Aspirin | 373 (89%) | 365 (87%) | 738 (88%) | 9,257 (85%) |
| Antiplatelet agents (excluding aspirin) | 46 (11%) | 61 (15%) | 107 (13%) | 1,591 (15%) |
| Statins | 306 (73%) | 316 (75%) | 622 (74%) | 8,096 (74%) |
| Angiotensin-converting enzyme inhibitors | 366 (87%) | 350 (84%) | 716 (85%) | 8,724 (80%) |
| Angiotensin II receptor blockers | 23 (5%) | 24 (6%) | 47 (6%) | 1,229 (11%) |
| β Blockers | 391 (93%) | 386 (92%) | 777 (93%) | 9,487 (87%) |
| Diltiazem or verapamil | 8 (2%) | 11 (3%) | 19 (2%) | 252 (2%) |
| Dihydropyridine calcium channel inhibitor | 37 (9%) | 35 (8%) | 72 (9%) | 1,110 (10%) |
| Nitrates | 193 (46%) | 176 (42%) | 369 (44%) | 4,733 (43%) |
| Diuretics (excluding antialdosterone) | 228 (54%) | 224 (53%) | 452 (54%) | 6,426 (59%) |
| Antialdosterone agents | 128 (30%) | 127 (30%) | 255 (30%) | 2,953 (27%) |
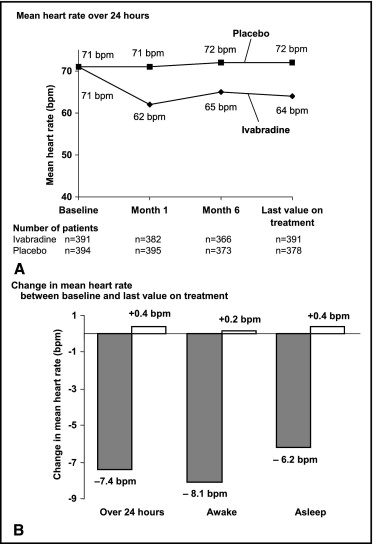
Treatment with ivabradine was associated with a decrease in mean 24-hour heart rate of 6.3 ± 9.5 beats/min (95% confidence interval 7.2 to 5.4) at last assessment ( Table 2 ) versus no change with placebo (0.4 ± 7.2 beats/min, 95% confidence interval −0.4 to 1.1, p <0.001, between-group difference). Decrease in heart rate after 1 month was maintained at 6 months and at last assessment ( Figure 2 ). Ivabradine-associated decrease in heart rate was greater during the waking hours than during sleep ( Figure 2 and Table 2 ).
| Variable | Mean Heart Rate Recorded Over 24 Hours (beats/min) | Mean Heart Rate Recorded When Awake (beats/min) | Mean Heart Rate Recorded When Asleep (beats/min) | |||
|---|---|---|---|---|---|---|
| Ivabradine | Placebo | Ivabradine | Placebo | Ivabradine | Placebo | |
| (n = 408) | (n = 399) | (n = 408) | (n = 399) | (n = 408) | (n = 399) | |
| Baseline | 70.6 ± 9.6 | 71.3 ± 9.2 | 74.1 ± 10.2 | 74.8 ± 10.0 | 63.7 ± 9.3 | 64.5 ± 8.6 |
| Last value | 64.3 ± 9.0 | 71.6 ± 9.4 | 67.2 ± 9.5 | 75.1 ± 10.2 | 58.5 ± 8.9 | 64.8 ± 9.0 |
| Change from baseline to last value | –6.3 ± 9.5 ⁎ | 0.4 ± 7.2 | –6.8 ± 10.4 | 0.3 ± 8.1 | –5.2 ± 8.9 | 0.4 ± 7.0 |
⁎ p <0.001 versus placebo (parametric covariance analysis, adjusted for country, β-blocker intake at randomization, and baseline value).
Incidence of episodes of heart rate <30 beats/min when awake or asleep was low ( Tables 3 and 4 ). Although there were more patients with a heart rate <40 or <50 beats/min when awake with ivabradine than with placebo (p <0.0001, between-group difference for <40 beats/min at last assessment), there was no difference in severity of episodes between groups (for <40 beats/min, median number of episodes at last assessment, 9 vs 6 episodes for ivabradine and placebo, respectively; median lowest heart rate registered 38 vs 37 beats/min). Similar observations were made for episodes of heart rate <40 beats/min when asleep.
| Variable | Ivabradine | Placebo | ||||
|---|---|---|---|---|---|---|
| Baseline | Month 1 | Last Value on Treatment | Baseline | Month 1 | Last Value on Treatment | |
| (n = 408) | (n = 395) | (n = 378) | (n = 399) | (n = 382) | (n = 391) | |
| Lowest heart rate (beats/min) | ||||||
| <50 | 152 (37%) | 294 (74%) | 255 (68%) | 136 (34%) | 129 (34%) | 140 (36%) |
| <40 | 15 (4%) | 57 (14%) | 46 (12%) | 11 (3%) | 10 (3%) | 14 (4%) |
| <30 | 1 (0.3%) | 2 (0.5%) | 4 (1%) | 2 (0.5%) | 2 (0.5%) | 2 (0.5%) |
| Sinus pause (seconds) | ||||||
| >2 | 9 (2%) | 26 (7%) | 27 (7%) | 13 (3%) | 11 (3%) | 16 (4%) |
| >2.5 | 2 (0.5%) | 5 (1%) | 5 (1%) | 4 (1%) | 2 (0.5%) | 4 (1%) |
| >3 | 1 (0.3%) | 3 (0.8%) | 3 (0.8%) | 1 (0.3%) | 1 (0.3%) | 2 (0.5%) |
| Atrioventricular block | ||||||
| Mobitz I | 3 (0.7%) | 3 (0.8%) | 5 (1%) | 6 (2%) | 3 (0.8%) | 4 (1%) |
| Mobitz II | 3 (0.7%) | 2 (0.5%) | 2 (0.5%) | 2 (0.5%) | 1 (0.3%) | 1 (0.3%) |
| Third degree | 0 (0%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pause due to atrioventricular block (seconds) | ||||||
| >2 | 4 (1%) | 3 (0.8%) | 5 (1%) | 7 (2%) | 2 (0.5%) | 5 (1%) |
| >2.5 | 1 (0.3%) | 2 (0.5%) | 2 (0.5%) | 1 (0.3%) | 0 (0%) | 3 (0.8%) |
| >3 | 1 (0.3%) | 1 (0.3%) | 2 (0.5%) | 1 (0.3%) | 0 (0%) | 2 (0.5%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


