Clopidogrel hypersensitivity affects up to 6% of treated patients, often leading to discontinuation of the drug. Conventional desensitization protocols incorporate a washout period off medication that may be problematic after percutaneous coronary intervention because premature discontinuation of dual antiplatelet therapy is a major risk factor for stent thrombosis. The purpose of this study was to evaluate a strategy for treating clopidogrel hypersensitivity without drug interruption using corticosteroids and antihistamines to facilitate development of physiologic tolerance. The study population consisted of 25 consecutive patients who developed clopidogrel hypersensitivity after percutaneous coronary intervention and were managed with suppressive therapy using corticosteroids and antihistamines. Treatment success (resolution of hypersensitivity symptoms without interrupting clopidogrel) was assessed, in addition to duration of clopidogrel therapy and adverse cardiac events during late follow-up (mean 670 ± 630 days). The cohort included 19 men and 6 women with a mean age of 62 ± 9 years. Drug-eluting stents were used in 16 patients (64%). Clopidogrel hypersensitivity occurred 6 ± 2 days after drug initiation. Treatment included corticosteroids (5 patients), antihistamines (5 patients), or corticosteroids and antihistamines (15 patients). Patients treated with corticosteroids received tapering courses for a mean of 10 ± 8 days. Treatment was successful with sustained symptom resolution in 22 of 25 patients (88%). Clopidogrel therapy was continued in successfully desensitized patients for 417 ± 369 days and in patients with drug-eluting stents for 529 ± 376 days. There were no deaths, myocardial infarctions, or stent thrombosis during extended follow-up. In conclusion, clopidogrel hypersensitivity can be successfully treated using short-course corticosteroids and antihistamines without interrupting drug therapy. This technique enables long-term continuation of clopidogrel and confers a low risk of adverse cardiac events.
Prolonged clopidogrel therapy is vital for the prevention of life-threatening stent thrombosis after percutaneous coronary intervention (PCI). Clopidogrel hypersensitivity, often manifesting as a pruritic rash, affects up to 6% of patients and results in premature drug discontinuation in 1.5% of patients. Hypersensitivity usually occurs during the first 14 days of therapy, when antiplatelet therapy is most critical after PCI. Clopidogrel desensitization protocols similar to those used for aspirin have been described with success rates of approximately 90%. To allow hypersensitivity symptoms to resolve before performing desensitization, these protocols require a drug washout period during which time patients are at risk for stent thrombosis while clopidogrel is withheld. The purpose of this study was to assess a strategy for treating clopidogrel hypersensitivity without drug interruption. Hypersensitivity symptoms were suppressed with short-term corticosteroids and antihistamines while continuing clopidogrel. The rationale of this strategy was to enable the development of physiologic tolerance without interrupting therapy, thus decreasing the risk for adverse cardiac events.
Methods
The study population was retrospectively identified as having developed clopidogrel hypersensitivity after undergoing cardiac catheterization with PCI from January 1, 2005 through December 30, 2008. Eligible subjects were maintained on clopidogrel while receiving suppressive therapy for an allergic reaction. Clopidogrel hypersensitivity was clinically diagnosed based on timing and characteristics of symptoms being related to initiation of clopidogrel therapy. Patients were excluded if clopidogrel therapy was unable to be reasonably linked to onset of hypersensitivity or was interrupted.
Medical records from inpatient hospitalization and follow-up visits were reviewed to determine duration of clopidogrel therapy, symptom recurrence, and incidence of adverse cardiac events. Clopidogrel hypersensitivity was treated with antihistamines and/or short-term corticosteroids, with the specific regimen according to the discretion of clinicians caring for a patient. Study protocol was reviewed and approved by the institutional review board at Thomas Jefferson University (Philadelphia, Pennsylvania).
The primary outcome was sustained resolution of hypersensitivity symptoms after the treatment regimen without discontinuation of clopidogrel. Secondary outcomes included recurrence of hypersensitivity reaction, ability to complete the minimum recommended duration of clopidogrel therapy, overall duration of clopidogrel therapy, and occurrence of major adverse cardiac events. Successful completion of clopidogrel therapy was defined as drug continuation beyond the minimum target duration of clopidogrel according to American College of Cardiology and American Heart Association guidelines at time of stent implantation as follows: 1 month for all bare metal stents, 3 months for sirolimus-eluting stents (SES) through December 2007, 6 months for paclitaxel-eluting stents (PES) through December 2007, and 12 months for SES or PES after December 2007. Descriptive statistics were used to analyze continuous and categorical data.
Results
The study population consisted of 25 patients with clopidogrel hypersensitivity after PCI who received suppressive therapy. Average age was 62 ± 9 years and 76% of patients were men ( Table 1 ). Multiple cardiovascular risk factors were present in 88% of patients. Prevalence of atopic illness in the form of a history of asthma, allergic rhinitis, or seasonal allergies was more frequent than found in the general population, occurring in 32% of subjects. Over 1/2 of patients had a known allergy to ≥1 other medication. Indications for cardiac catheterization included an acute coronary syndrome in 60%, stable angina in 4%, and positive screening test result (stress or coronary computed tomographic angiography) in 36%. Patients received a mean of 2.2 stents with drug-eluting stents used in 64% (12% PES, 52% SES).
| Variable | |
|---|---|
| Age (years) | 62 ± 9 |
| Men/women | 19 (76%)/6 (24%) |
| History of hypertension | 19 (76%) |
| History of hyperlipidemia | 18 (72%) |
| Previous percutaneous coronary intervention or coronary artery bypass grafting | 9 (36%) |
| Previous myocardial infarction | 5 (20%) |
| Diabetes mellitus | 6 (24%) |
| Peripheral vascular disease | 3 (12%) |
| Cardiomyopathy | 2 (8%) |
| Stroke | 1 (4%) |
| Atopic history | |
| Asthma | 3 (12%) |
| Allergic rhinitis | 3 (12%) |
| Seasonal allergies | 5 (20%) |
| Medication allergies | 14 (56%) |
| Indication | |
| ST-segment elevation myocardial infarction | 3 (12%) |
| Non–ST-segment elevation myocardial infarction | 5 (20%) |
| Unstable angina pectoris | 7 (28%) |
| Stable angina pectoris | 1 (4%) |
| Abnormal stress test result | 7 (28%) |
| Abnormal computed tomographic angiogram | 2 (8%) |
| Multiple stents placed | 17 (68%) |
| Stent type | |
| Sirolimus-eluting stent | 13 (52%) |
| Paclitaxel-eluting stent | 3 (12%) |
| Bare metal stent | 9 (36%) |
Clinical presentation and treatment outcomes of each study patient are presented in Table 2 . Hypersensitivity reaction occurred on day 6 ± 2 of clopidogrel therapy. It is noteworthy that all cases of clopidogrel hypersensitivity developed within the first 10 days of therapy. The most common presentation of clopidogrel hypersensitivity was a pruritic rash starting on the trunk and spreading to the extremities (n = 20). Additional presentations included hives (n = 3), nonpruritic rash (n = 1), and angioedema (n = 1).
| Patient Number | Days to Onset | Reaction | Therapy | Duration of Clopidogrel |
|---|---|---|---|---|
| 1 | 6 | pruritic rash on torso, back | Solu-Medrol | >1,138 |
| 2 | 8 | rash on chest, back, arms, legs | diphenhydramine | 888 |
| 3 | 8 | pruritic rash chest, back, arms | prednisone, fexofenadine | 454 |
| 4 | 6 | confluent rash trunk, back, abdomen | prednisone, montelukast | >1,036 |
| 5 | 8 | pruritic rash on back | Solu-Medrol, diphenhydramine, fexofenadine, doxepin | 372 |
| 6 | 7 | pruritic rash on trunk, back | diphenhydramine, loratadine | 92 |
| 7 | 8 | pruritic rash chest, arms, legs | Solu-Medrol, diphenhydramine, fexofenadine | 91 |
| 8 | 10 | hives over entire body | Solu-Medrol, fexofenadine, montelukast | failed |
| 9 | 4 | pruritic rash and hives on chest, arms | Solu-Medrol, diphenhydramine, cetirizine, topical hydrocortisone | 396 |
| 10 | 5 | florid rash | Solu-Medrol | >382 |
| 11 | 7 | rash | Solu-Medrol | >177 |
| 12 | 1 | diffuse pruritus | diphenhydramine | >590 |
| 13 | 4 | diffuse pruritic rash | Solu-Medrol, diphenhydramine | >592 |
| 14 | 6 | angioedema, pruritic rash back, legs, arms | Solu-Medrol, diphenhydramine, famotidine | failed |
| 15 | 9 | rash | Solu-Medrol, diphenhydramine, | 165 |
| 16 | 7 | pruritic rash | Solu-Medrol, diphenhydramine, montelukast | failed |
| 17 | 3 | rash | diphenhydramine | >848 |
| 18 | 7 | nonpruritic rash ankles, shins | prednisone, fexofenadine, famotidine | >189 |
| 19 | 2 | pruritic rash | diphenhydramine, loratadine | >92 |
| 20 | 7 | pruritic rash | Solu-Medrol, diphenhydramine, hydroxyzine | >92 |
| 21 | 7 | pruritic rash chest, back, arms, legs | Solu-Medrol, diphenhydramine, loratadine | >848 |
| 22 | 1 | hives | prednisone, diphenhydramine, loratadine, famotidine | >58 |
| 23 | 7 | pruritic rash | Solu-Medrol | >287 |
| 24 | 7 | pruritic rash chest, back, arms, legs | fexofenadine, hydroxyzine | >9 |
| 25 | 5 | generalized urticaria | Solu-Medrol, diphenhydramine, loratadine, hydroxyzine | 30 |
Treatment of clopidogrel hypersensitivity consisted of antihistamines alone (n = 5), corticosteroids alone (n = 5), or combination therapy (n = 15). Antihistamines were used in the short-acting form (n = 7) or long-acting nonsedating form (n = 3), with 10 patients receiving both forms. The most commonly prescribed corticosteroid regimen was a 6-day Solu-Medrol taper (Medrol Dosepak; Pfizer, New York, New York). Overall mean duration of corticosteroid therapy was 10 ± 8 days. One patient required continued corticosteroid therapy for 30 days in the form of prednisone 5 mg for symptom suppression. Additional pharmacotherapies were used in 7 patients including leukotriene inhibitor, histamine-2 receptor blocker, or topical corticosteroid. Consultation with an allergist was obtained in 14 patients (56%).
Treatment of clopidogrel hypersensitivity was successful in 22 of 25 patients (88%). Most patients (72%) were treated as outpatients. All patients successfully developing tolerance were able to achieve the minimum target duration of clopidogrel therapy. Two patients had recurrent symptoms successfully treated with an additional longer course of corticosteroid or addition of a leukotriene inhibitor. Desensitization therapy failed in 3 patients owing to development of angioedema, desquamating rash, or intolerable pruritus in individual patients.
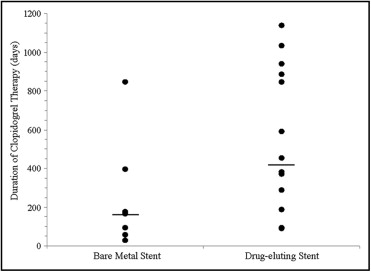
All 22 patients who were effectively desensitized went on to successfully complete their benchmark duration of clopidogrel therapy. Clopidogrel therapy duration uniformly exceeded the minimum target duration recommended by American College of Cardiology/American Heart Association guidelines at time of stent implantation ( Figure 1 ). Overall, patients received 417 ± 369 days of clopidogrel, with 222 ± 281 days in those with bare metal stents and 529 ± 376 days in those with drug-eluting stents. Clopidogrel therapy continues in 14 of 22 patients as of last follow-up with the longest ongoing duration of clopidogrel being 1,138 days.