We aim to evaluate the potential benefit and risk of addition of vorapaxar to standard medical therapy in patients who underwent coronary revascularization with either percutaneous coronary revascularization or coronary artery bypass graft surgery. We searched PubMed, EMBASE, the Cochrane Central Register of Controlled trials, and the clinical trial registry maintained at clinicaltrials.gov for randomized control trials evaluating the safety and efficacy of vorapaxar in patients who underwent coronary revascularization procedures with either percutaneous coronary revascularization or coronary artery bypass graft surgery. Event rates were compared using a Forest plot of relative risk using a random-effects model. The 5 studies (n = 24,025) that met all criteria were included in the final analysis. After coronary revascularization procedures, addition of vorapaxar to standard medical therapy was associated with reduction in the risk of myocardial infarction (MI; risk ratio 0.83 [0.75 to 0.92]) and ischemic stroke (0.011 [0.007 to 0.016]); however, it also resulted in significant increase risk of hemorrhagic stroke (1.57 [1.01 to 2.44]) and Thrombolysis In Myocardial Infarction major and minor bleeds (1.36 [1.07 to 1.70]). There was no significant difference in the risk of cardiovascular mortality (0.90 [0.73 to 1.09]), repeat revascularization (0.78 [0.23 to 2.70]), and stent thrombosis (0.95 [0.62 to 1.45]) in the vorapaxar and control groups. In conclusion, after coronary revascularization procedures, addition of vorapaxar to standard medical therapy was associated with reduction in the risk of MI and ischemic stroke and increase in risk of hemorrhagic stroke and Thrombolysis In Myocardial Infarction major and minor bleeds.
Recently, the Food and Drug Administration has approved vorapaxar for long-term secondary prevention of atherothrombotic events in stable patients with a previous myocardial infarction (MI) or peripheral arterial disease and without a history of stroke or transient ischemic attack. However, it remains largely unclear if addition of protease-activated receptor-1 (PAR1) antagonists would have an incremental benefit when added to current standard therapy in patients who underwent coronary revascularization procedures (percutaneous coronary revascularization [PCI] or coronary artery bypass graft surgery [CABG]), which is of particular interest, as this patient population is at increased risk of rethrombosis, especially stent thrombosis (ST) and graft occlusion, mediated by persistent platelet activating mechanisms. Hence, in the current meta-analysis, we evaluated the potential benefit and risk of addition of vorapaxar to standard in patients who underwent coronary revascularization with either PCI or CABG.
Methods
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement for reporting systematic reviews and meta-analyses of randomized control trials (RCTs) for the protocol of our meta-analysis. We searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and the clinical trial registries maintained at clinicaltrials.gov were searched for randomized trials evaluating the safety and efficacy of vorapaxar. The following search terms were used: vorapaxar, SCH530348, protease-activated receptor-1 inhibitor or PAR-1 antagonist, coronary revascularization, percutaneous coronary intervention, PCI, coronary artery revascularization surgery, CABG. Pertinent trials were also searched in major national/international cardiology meetings (American College of Cardiology, American Heart Association, European Society of Cardiology, and Transcatheter Cardiovascular Therapeutics). References of original and review studies were crosschecked. We manually searched references of identified studies. The search period took place from January1, 2002, to September 30, 2015. No language restrictions were applied. Prospective RCTs were selected if they met following criteria: (1) study population of patients who underwent PCI and/or CABG; (2) assignment of participants to vorapaxar or a placebo group; and (3) provide data on outcomes for vorapaxar and placebo arms. Non-RCTs were excluded.
Two reviewers (AS, SG) independently screened the titles and abstracts for relevance. The manuscripts of selected titles/abstracts were reviewed for inclusion or exclusion using the previously mentioned selection criteria. Two reviewers (AS, AG) independently determined the studies to be included and excluded, and data from the relevant studies were extracted using predefined extraction forms. Any disagreements in data extraction were discussed until consensus was reached. Key study and patient characteristics were extracted, including the following outcomes, reported at the longest available follow-up according to intention-to-treat principles: all-cause mortality, cardiac mortality, MI, ST, target vessel revascularization, Thrombolysis In Myocardial Infarction (TIMI) non-CABG major bleeding, ischemic stroke, and intracranial hemorrhage (ICH). The statistical analysis was performed according to the recommendations from the Cochrane Collaboration using Review Manager, version 5.1 (The Nordic Cochrane Center, The Cochrane Collaboration, 2008, Copenhagen). A random-effects model with inverse variance weighting was used to calculate risk ratio and 95% CI, associated with vorapaxar versus placebo for previously mentioned end points. Forest plot was used to observe the overall effect of studies. Heterogeneity between studies was assessed using the Cochrane’s Q test and I 2 statistic, which denotes the percentage of total variation across studies that are a result of heterogeneity rather than chance. Heterogeneity was considered significant if the p value was <0.05. Publication bias was assessed by the Begg’s test and Egger’s regression test.
Results
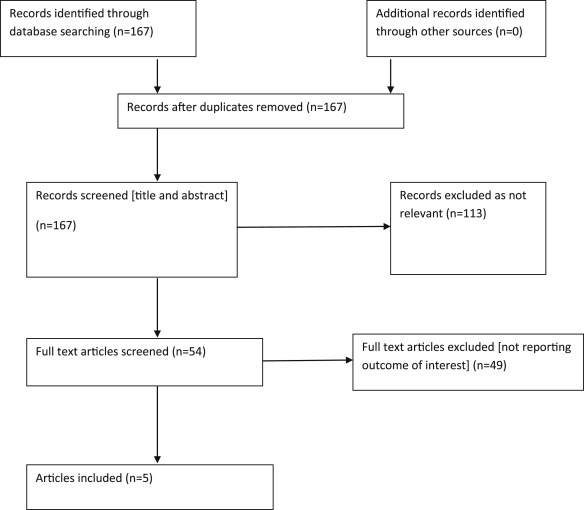
Five studies (n = 24,025) are selected for final analysis ( Figure 1 ). Baseline characteristics of patients included in the studies included in current analysis have been summarized in Table 1 . Clinical outcomes with the addition of vorapaxar versus placebo to standard-of-care practices after PCI or CABG are summarized in Figures 2 to 8 and in Table 2 .
| STUDY | No. | Follow up (in days) | Age (yr) | Women | Weight (kg) | DM | HTN | HLD | PAD | Previous MI | Previous PCI | Previous CABG | Previous CVA | Aspirin | Plavix | Heparin | Bivalirudin | GPIIb/IIIA inhibitors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Becker 2009 ∗ | 151 | 60 | 62.7+9.3 | 15% | 90.16+18.7 | 32% | – | – | – | 44% | 45% | – | – | 98% | 97% | 40% | 50% | 5% |
| Becker2009 † | 422 | 60 | 64.5+9.8 | 26% | 89.2+19.2 | 36% | – | – | – | 41% | 49% | – | – | 99% | 97% | 43% | 46% | 9% |
| Becker 2009 ‡ | 106 | 60 | 61.5+9.6 | 26% | 93+20.3 | 26% | – | – | – | 25% | 34% | – | – | 86% | 28% | 20% | 5% | 0% |
| Becker 2009 § | 351 | 60 | 62.1+9.0 | 34% | 91.2+21.1 | 31% | – | – | – | 27% | 31% | – | – | 89% | 32% | 26% | 2% | 1% |
| Goto 2010 ¶ | 21 | 60 | 65+11 | 24% | 67+12 | 52% | 81% | – | – | 10% | 100% | – | – | 100% | – | 100% | – | – |
| Goto 2010 ‖ | 71 | 60 | 64+9 | 20% | 65+10 | 52% | 75% | – | – | 13% | 93% | – | – | 100% | – | 100% | – | – |
| Tricoci 2012 ‡‡ | 6471 | 502 | 64 | 28% | 80 | 31.4% | 71% | 62.2% | 7.2% | 29.2% | 23.7% | 11.8% | 4.1% | 96.9% | 87.1% | – | – | – |
| Tricoci2012 §§ | 6473 | 502 | 64 | 28% | 80.4 | 31.5% | 70.1% | 62.4% | 7.2% | 29.4% | 24.1% | 12% | 4.5% | 96.4% | 87.6% | – | – | – |
| Morrow 2012 ¶¶ | 13224 | 912 | 61 | 24% | – | 25.4% | 69% | 83.3% | 22.3% | 67.2% | – | – | 23.7% | 93.5% | 62% | – | – | – |
| Morrow 2012 ‖‖ | 13225 | 912 | 61 | 24% | – | 25.5% | 68.4% | 83.1% | 21.9% | 67.3% | – | – | 23.7% | 93.6% | 62% | – | – | – |
† VORAPAXAR Primary PCI cohort.
‡ PLACEBO Secondary non PCI cohort.
§ VORAPAXAR secondary non PCI cohort.
‖ VORAPAXAR Primary PCI cohort.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


