Observational studies strongly associate vitamin D deficiency with a variety of cardiovascular diseases beyond defects in bone and calcium metabolism. Vitamin D has multiple mechanisms that potentially may affect cardiovascular health. Because vitamin D deficiency is common, therapies directed at the replacement of vitamin D may be beneficial. To date however, studies evaluating vitamin D supplementation are few and have not consistently shown benefit. It is possible that the lack of benefit in these studies may have arisen from suboptimal levels of vitamin D supplementation or other unknown factors. Nevertheless, the growing body of observational data and consistent findings of relatively high rates of low vitamin D serum levels warrant further well-designed studies to investigate the relation between vitamin D and cardiovascular health. In conclusion, vitamin D is now recognized as important for cardiovascular health and its deficiency as a potential risk factor for several cardiovascular disease processes.
Cardiovascular disease is the most common cause of mortality and morbidity in the United States and many other nations. A growing body of evidence suggests a possible association between vitamin D deficiency and many cardiovascular disorders, including hypertension, peripheral vascular disease, diabetes mellitus, metabolic syndrome, coronary artery disease, and heart failure. In this review, we focus on current evidence, potential mechanisms, and the possible role of vitamin D supplementation in cardiac health.
Metabolism of Vitamin D
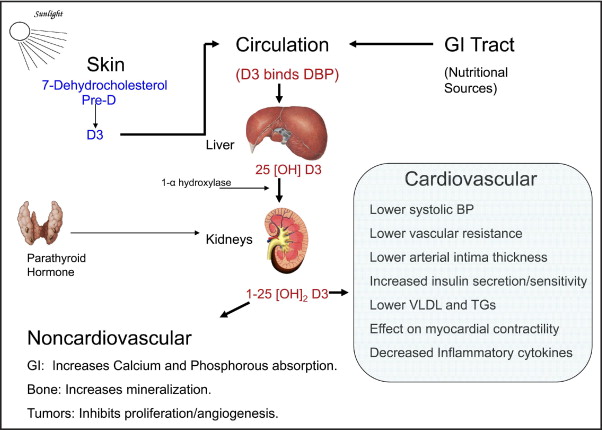
Vitamin D belongs to a group of secosteroid molecules that are traditionally associated with bone and calcium metabolism. Although 5 forms of vitamin D (D 1 through D 5 ) are known, vitamins D 2 and D 3 are the most studied forms ( Table 1 ). Ergocalciferol, or vitamin D 2 , is principally synthesized in plants and invertebrates. It is typically consumed in the human diet and as supplements or fortified products. Cholecalciferol, or vitamin D 3 , is mainly of vertebrate animal origin and commonly consumed in the form of oily fish. Cholecalciferol is also synthesized in human skin after exposure of 7-dehydrocholesterol to solar ultraviolet radiation. Endogenous and consumed vitamin D is stored in fat tissues and released into the circulation ( Table 1 ). Vitamin D 3 is bound to a circulating glycoprotein called vitamin D–binding protein ( Figure 1 ). The liver converts vitamin D to 25-hydroxy (25[OH]) vitamin D. In a rate-limiting step, the kidneys convert 25(OH) vitamin D to its active form 1,25(OH) 2 vitamin D ( Figure 2 ).
| Class | Chemical Composition | Significance |
|---|---|---|
| Vitamin D 1 | Combination of ergocalciferol and lumisterol | |
| Vitamin D 2 | Ergocalciferol: made from ergosterol or pre–vitamin D 2 | Made by invertebrates, fungus, and plants in response to ultraviolet irradiation; not made by vertebrates |
| Vitamin D 3 | Cholecalciferol: made from 7-dehydrocholesterol or pre–vitamin D 3 | Made in the skin as a response to ultraviolet B radiation after reacting with 7-dehydrocholesterol |
| Vitamin D 4 | Dihydroergocalciferol: vitamin D 2 without 22,23 double bond | Ineffective form of vitamin D |
| Vitamin D 5 | Sitocalciferol: made from 7-dehydrositosterol | May have antitumor properties |
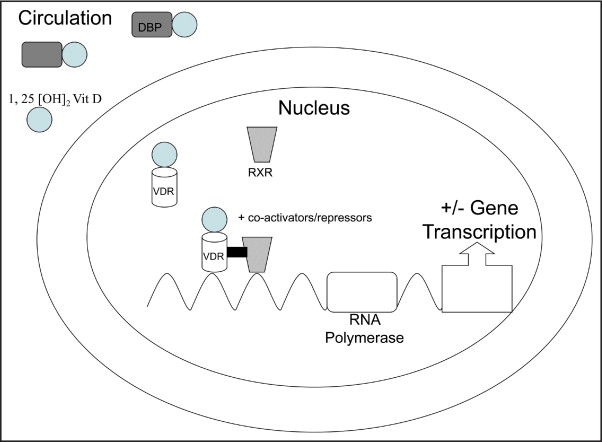
The active form of vitamin D (1,25[OH] 2 vitamin D) binds to vitamin D receptors (VDR). The conjugated vitamin D with its receptor forms a heterodimer complex with retinoid X receptor. Together with several other factors and an activator, this complex attaches to vitamin D–responsive elements on deoxyribonucleic acid and alters gene expression ( Figure 1 ). VDRs are prominently present in enterocytes, osteoblasts, parathyroid gland cells, and distal renal tubule cells. Vitamin D increases calcium absorption from gut and renal tubular cells and suppresses parathyroid hormone. It acts on osteoblasts and increases bone mineral density. Recent investigations have also shown a significant presence of VDR in the liver, the immune system, and skeletal and cardiac muscle.
Sources of Vitamin D and Normal Serum Levels
Cutaneous synthesis of vitamin D 3 from sunlight exposure is the major source (80% to 90%) of vitamin D in humans under natural conditions. Total-body sun exposure to 1 minimal erythemal dose while wearing a bathing suit provides the equivalent of 250 to 500 μg (10,000 to 20,000 IU) of vitamin D per day. The dietary supply of vitamin D is minor compared to cutaneous formation but can become an important source of vitamin D with supplementation. Oily fish such as salmon, mackerel, herring, and sardines are rich sources of vitamin D. Fortified milk and juices are fortified to provide ≥100 IU per 8-oz serving in the United States and Canada. Ordinary dietary sources usually provide 2.5 μg (100 IU) of vitamin D per day, and fortified foods may provide up to 5 to 10 μg (200 to 400 IU) of vitamin D daily.
Serum 25(OH) vitamin D is the major circulating metabolite of vitamin D and reflects vitamin D input from cutaneous synthesis and dietary intake. Serum 1,25(OH) 2 vitamin D levels can be normal or even elevated in patients with vitamin D deficiency. The most beneficial serum concentrations of 25(OH) vitamin D are observed at ≥30 ng/ml (≥75 nmol/L). Most experts agree that vitamin D insufficiency is present with 25(OH) vitamin D levels of 21 to 29 ng/ml, while levels <20 ng/ml (<50 nmol/L) indicate vitamin D deficiency.
Sources of Vitamin D and Normal Serum Levels
Cutaneous synthesis of vitamin D 3 from sunlight exposure is the major source (80% to 90%) of vitamin D in humans under natural conditions. Total-body sun exposure to 1 minimal erythemal dose while wearing a bathing suit provides the equivalent of 250 to 500 μg (10,000 to 20,000 IU) of vitamin D per day. The dietary supply of vitamin D is minor compared to cutaneous formation but can become an important source of vitamin D with supplementation. Oily fish such as salmon, mackerel, herring, and sardines are rich sources of vitamin D. Fortified milk and juices are fortified to provide ≥100 IU per 8-oz serving in the United States and Canada. Ordinary dietary sources usually provide 2.5 μg (100 IU) of vitamin D per day, and fortified foods may provide up to 5 to 10 μg (200 to 400 IU) of vitamin D daily.
Serum 25(OH) vitamin D is the major circulating metabolite of vitamin D and reflects vitamin D input from cutaneous synthesis and dietary intake. Serum 1,25(OH) 2 vitamin D levels can be normal or even elevated in patients with vitamin D deficiency. The most beneficial serum concentrations of 25(OH) vitamin D are observed at ≥30 ng/ml (≥75 nmol/L). Most experts agree that vitamin D insufficiency is present with 25(OH) vitamin D levels of 21 to 29 ng/ml, while levels <20 ng/ml (<50 nmol/L) indicate vitamin D deficiency.
Epidemiology of Vitamin D Deficiency
Vitamin D, under ideal conditions, is probably not required in the diet, because most mammals including humans can synthesize it from direct sunlight exposure. However, worldwide, most humans typically expose ≤5% of their skin to infrequent periods of unshielded sunlight, a behavior that commonly leads to vitamin D deficiency. This is far less solar exposure than that experienced in most historical human cultures and among free-living primates. In 1 study, 36% of young healthy free-living adults in the United States aged 18 to 29 years had vitamin D deficiency at the end of winter. In elderly and institutionalized patients, the prevalence of vitamin D deficiency is higher. The Third National Health and Nutrition Examination Survey (NHANES III) reported the prevalence of vitamin D deficiency in United States adults to be 25% to 57%. Risk factors for vitamin D deficiency include advanced age, dark skin color, institutionalized or homebound status, increased distance from the equator, winter season, clothing, the use of sunscreen, air pollution, smoking, obesity, malabsorption, renal disease, liver disease, and medications.
Recommended Daily Intake, Toxicity, and Assay of Vitamin D
The present recommended daily dose of vitamin D is 400 IU. It has been estimated that for every 100 IU of vitamin D ingested, the blood level of 25(OH) vitamin D increases by 1 ng/ml (2.5 nmol/L). For many individuals, the present recommended daily allowance may not be sufficient to achieve optimal serum concentrations of vitamin D. African Americans with low sun exposure require 2,100 to 3,100 IU/day of oral vitamin D throughout the year to achieve a serum 25(OH) vitamin D concentration of ≥30 mg/mL (≥75 nmol/L). Many experts now recommend a daily vitamin D dose of 1,000 to 2,000 IU for most individuals, with higher doses of vitamin D (up to several doses of 50,000 IU) required initially to treat hypovitaminosis.
Hypervitaminosis D, which is extremely rare and typically is caused by prolonged massive doses of oral supplementation, presents with symptoms related to hypercalcemia, including anorexia, nausea, and vomiting, followed by polyuria, polydipsia, weakness, nervousness, pruritus, and eventually even renal failure. Overall, however, evidence suggests that vitamin D is well tolerated over a large intake range. Case reports have shown that 150,000 IU of vitamin D daily for 28 years, and serum concentrations up to 1,126 nmol/L for months to years, did not result in significant hypercalcemia side effects.
Currently, several methods of vitamin D assay are available. Liquid chromatography tandem mass spectroscopy measures all forms of circulating 25(OH) vitamin D and is considered the “gold standard.” Other simpler methods, such as radioimmunoassay, enzyme-linked immunoassay, and chemiluminescence assay, may not measure all circulating forms of vitamin D. Clinicians must be aware of the assay methods and normal ranges for their laboratories.
Clinical Conditions Associated With Vitamin D Deficiency
A wide range of cardiovascular disease states have been associated with vitamin D deficiency involving multiple potential mechanisms ( Table 2 ).
| Pathology | Proposed Mechanism of Action |
|---|---|
| Hypertensive vascular disease |
|
| Peripheral vascular disease |
|
| Diabetes mellitus |
|
| Lipid metabolism |
|
| Coronary artery disease |
|
| Heart failure |
|
| Arrhythmias |
|
Vitamin D and hypertensive vascular disease
Essential hypertension is a major risk factor for cardiovascular disease. Vitamin D appears to be related to blood pressure control via multiple pathways and is inversely related to serum renin activity. Similarly, a decrease in blood pressure was seen in subjects who were exposed to ultraviolet B radiation. The effects of vitamin D on the suppression of renin activity may be due to increased intracellular calcium levels. Vitamin D replacement in deficient subjects significantly improved flow-mediated dilatation of the brachial artery, suggesting a role of vitamin D in the sensitivity of vascular smooth muscle cells.
Initial small retrospective observational studies have shown significant inverse correlations between vitamin D levels and systolic blood pressure. In a small study from Belgium of 25 patients with hypertension, vitamin D levels were inversely correlated with systolic blood pressure, diastolic blood pressure, and calf vascular resistance. In a subsequent study involving normotensive men, a similar inverse correlation between 125(OH) 2 vitamin D and systolic blood pressure was observed. Conversely, in another study, vitamin D levels did not differ between newly diagnosed patients with hypertension and their matched controls.
Data from a large cross-sectional national study involving a noninstitutionalized population aged >20 years, NHANES III, were used to evaluate the relation between serum 25(OH) vitamin D concentrations and blood pressure. After excluding those who were taking antihypertensive medications, a total of 12,644 patients were included in the analysis. The mean blood pressure varied inversely with serum 25(OH) vitamin D levels, with the association remaining significant after adjustment for age, gender, race or ethnicity, and physical activity. The impact of vitamin D deficiency in the elderly (age >50 years) was highly significant (p = 0.021).
Interventions with vitamin D replacement were attempted to support the hypothesis that changes in vitamin D status affect blood pressure. In a randomized study, women aged >70 years with 25(OH) vitamin D levels <20 ng/ml were randomly assigned to receive supplementation with calcium 1,200 mg/day or calcium 1,200 mg/day plus vitamin D (cholecalciferol) 800 IU/day. Within 8 weeks of treatment, systolic blood pressure in the vitamin D–treated group had decreased by an average of 13 mm of Hg (p = 0.02). In a similar randomized study involving patients with diabetes mellitus and serum 25(OH) vitamin D levels <20 ng/ml, patients were randomly assigned to receive a 1-time dose of ergocalciferol 100,000 IU or placebo. Vitamin D supplementation produced a significant decrease in systolic blood pressure. Similar benefits of vitamin D on blood pressure were noticed in other small studies. However, a recent meta-analysis provided little support for a positive effect of vitamin D supplementation on blood pressure.
Vitamin D and peripheral vascular disease
Vitamin D levels have been inversely correlated with calf vascular resistance and positively correlated with calf blood flow. Similar associations were identified in the NHANES III study. After multivariate adjustment for demographics, co-morbidities, physical activity level, and laboratory measures, low 25(OH) vitamin D levels were associated with a higher prevalence of peripheral arterial disease. Vitamin D deficiency is also strongly associated with increased thickness of the intima-media in carotid arteries. One third of the excess risk for peripheral arterial disease in an African American population was attributed to racial differences in vitamin D status. Similarly, a high prevalence of vitamin D deficiency with secondary hyperparathyroidism was observed in a nondiabetic population with peripheral vascular disease. To date, no interventional studies have examined the specific effect of vitamin D replacement on peripheral vascular disease.
Vitamin D and diabetes mellitus
Pancreatic β-cell dysfunction, peripheral tissue resistance to insulin, and chronic inflammation appear to be possible mechanisms for the role of vitamin D in diabetes mellitus. VDRs have been found in pancreatic islets, indicating a possible role for vitamin D in insulin secretion. Basal insulin secretion rate was not altered in VDR-knockout mice, but insulin secretion rate after a challenge with glucose diet was impaired in vitamin D deficiency. Vitamin D may affect intracellular calcium levels in pancreatic cells, which is an important stimulus for insulin secretion. In peripheral tissues, VDRs were found in skeletal muscles and adipose tissue. Vitamin D also has been shown to control insulin receptor expression and insulin responsiveness for glucose transport, establishing its role in insulin secretion and sensitivity.
Observational human studies examined the seasonal and geographic variation of type 1 diabetes mellitus and its relation to vitamin D deficiency. The European Community Concerted Action on the Epidemiology and Prevention of Diabetes (EURODIAB) study found a 33% reduction in the risk for developing childhood-onset type 1 diabetes in children who received vitamin D supplementation. Seasonal variations of glycemic control attributed to vitamin D level fluctuations were reported. Replacing vitamin D improves insulin secretion, peripheral insulin sensitivity in patients with type 2 diabetes, and glycosylated hemoglobin levels. Metabolic syndrome is also prevalent in patients with vitamin D deficiency.
Although the data from observational studies are strong, the expected benefit from replacement of vitamin D on fasting blood glucose, glucose tolerance, or insulin sensitivity was not observed in some studies. This variance may be due to ethnic differences or VDR gene polymorphisms. Nevertheless, 2 meta-analyses pooling large amounts of data suggest that vitamin D may have a role in the prevention of type 2 diabetes mellitus.
Vitamin D and lipid metabolism
Serum levels of 1,25(OH) 2 vitamin D are inversely correlated with very low density lipoprotein and triglyceride levels. Vitamin D deficiency may cause an abnormal lipid profile by increasing peripheral insulin resistance and contributing to metabolic syndrome. However, oral supplements of vitamin D 3 in postmenopausal women did not improve total cholesterol, low-density lipoprotein, or high-density lipoprotein levels over 12 months. Studies have suggested that statin therapy may increase vitamin D levels, a finding that may account for some of the nonlipid pleiotropic actions of statins. It is postulated that the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase enzyme by statins results in increased 7-dehydrocholesterol. This excess 7-dehydrocholesterol is then converted to 25-hydroxycholecalciferol by sunlight or the CYP11A1 enzyme, thereby increasing vitamin D levels ( Figure 2 ). Last, a recent study that examined reductions in VDR signaling in patients with diabetes found increased foam cell formation in macrophages, an early sign of atherosclerosis.
Vitamin D and coronary artery disease
As discussed previously, many coronary risk factors, including hypertension, diabetes mellitus, and lipid levels, are affected by vitamin D. Vitamin D has also been shown to affect endothelial function and decrease vascular calcification. Calcification of coronary arteries was inversely correlated with vitamin D levels. Earlier observations in the 1980s and 1990s found geographic and seasonal differences in mortality from ischemic heart disease. The initial suggestion of vitamin D as a protective factor came from a study in the United Kingdom showing that mortality from ischemic heart disease was inversely proportional to the hours of sunlight.
Larger cross-sectional observations using the NHANES databases found that in a sample of 16,603 men and women aged >18 years, those with ischemic heart disease and stroke had a greater frequency of 25(OH) vitamin D deficiency (p <0.0001). This was confirmed by a more recent analysis involving 8,351 adults in which the prevalence of vitamin D deficiency was 74% in patients with coronary artery disease and heart failure. In the general population, the lowest quartile of 25(OH) vitamin D level (<17.8 ng/ml) was independently associated with all-cause mortality. Together, the findings of these epidemiologic studies suggest that poor vitamin D status is associated with poor cardiovascular outcomes.
Multiple studies evaluated the relation of vitamin D prospectively with long-term cardiovascular outcomes in subjects with no histories of cardiovascular disease. In dialysis patients, those who were vitamin D deficient were at significantly increased risk for early mortality. Similarly, in healthy male health professionals aged 40 to 75 years with no histories of coronary artery disease, vitamin D deficiency (25[OH] vitamin D <15 ng/ml) was associated with a twofold increased rate of myocardial infarction over a 10-year period. In the Framingham Offspring Study, subjects with no histories of cardiovascular disease and severe vitamin D deficiency (25[OH] vitamin D <10 ng/ml) had increased risk for developing a first cardiovascular event after 5 years of follow-up compared with subjects with of 25(OH) vitamin D levels >15 ng/ml (hazard ratio 1.80, 95% confidence interval 1.05 to 3.08). In >3,000 subjects who underwent coronary angiography, those with severe vitamin D deficiency (<10 ng/ml) had 3 to 5 times the risk for death from sudden cardiac death, heart failure, or fatal stroke during a 7-year follow-up period compared to those who had optimal levels (>30 ng/ml).
Although the significance of vitamin D deficiency in coronary artery disease is well established in observational studies, few studies have been conducted to evaluate the impact of vitamin D supplementation on the risk for cardiovascular mortality. In the Women’s Health Initiative (WHI) trial, postmenopausal women were randomized to vitamin D 400 IU plus calcium 1,000 mg daily supplementation or placebo and followed for 7 years. The supplements had no significant effect on mortality rates. Elderly subjects (aged 65 to 85 years) living in the community were given 100,000 IU oral vitamin D 3 supplementation or matching placebo every 4 months over 5 years. Despite supplementation, the relative risk for total mortality did not change (odds ratio 0.88, 95% confidence interval 0.74 to 1.06, p = 0.18).
Vitamin D and heart failure
The major potential mechanisms that may explain a direct protective effect of vitamin D against heart failure include effects on myocardial contractile function, regulation of natriuretic hormone secretion, effects on extracellular matrix remodeling, reduced left ventricular hypertrophy, and the regulation of inflammatory cytokines ( Figure 2 ). Indirectly, vitamin D can also affect cardiac function by altering parathyroid hormone and serum calcium levels. The initial evidence in humans came from dialysis patients. In patients with uremic cardiomyopathy, treatment with 1-α hydroxyl cholecalciferol 1 μg/day for 6 weeks produced a decrease in plasma parathyroid concentration and an increase in fractional fiber shortening on M-mode echocardiography (p <0.025).
Observational studies have shown that osteoporosis, osteopenia, and low serum 25(OH) vitamin D levels are common in patients with congestive heart failure. It has been noted that there is ethnic variation in the incidence of heart failure and serum vitamin D levels. A case-control study demonstrated that patients with heart failure and controls differed in several vitamin D–associated lifestyle factors, such as urban dwelling, sports club membership, and the number of summer holidays during earlier life periods. In a study involving African American patients with left ventricular ejection fractions <35%, vitamin D deficiency (≤30 ng/ml) was associated with decompensated heart failure and prolonged hospital stays.
Heart failure in African American patients is a unique disease that is characterized by greater incidence, disease severity, and overall mortality compared to other populations. Serum parathyroid hormone levels and vitamin D status may explain some of the differences. Hyperparathyroidism is associated with left ventricular hypertrophy, cardiomyopathy and increased mortality. Vitamin D deficiency leads to increased secretion of parathyroid hormone and activation of the rennin-angiotensin-aldosterone and immune systems. Serum 25(OH) vitamin D levels are inversely correlated with serum parathyroid hormone levels. African American patients are also at risk for developing hyperparathyroidism and cardiomyopathy from other mechanisms, including secondary renin-angiotensin-aldosterone system activation and the use of loop diuretics. Observational studies have shown that vitamin D deficiency and hyperparathyroidism are more common in African American patients and universally present in patients with heart failure. Studies have also shown that 30% of African American women remain vitamin D deficient despite oral supplementation. Unrecognized, untreated, or partially treated vitamin D deficiency and associated hyperparathyroidism in African Americans might explain some of their morbidity and mortality associated with heart failure.
Low vitamin D levels were associated with poor outcomes in patients with end-stage heart failure awaiting heart transplantation. A study on VDR gene polymorphisms in patients with end-stage renal disease compared to normal subjects suggested that vitamin D signaling is implicated in the regulation of left ventricular mass and hypertrophy in end-stage renal disease (p <0.001). Hemodialysis patients with secondary hyperparathyroidism when treated with intravenous vitamin D showed significant reductions in left ventricular wall thickness and left ventricular mass index. Similarly vitamin D supplementation reduced inflammatory markers in patients with heart failure and improved serum parathyroid hormone levels. However, there was no significant direct survival benefit with vitamin D supplementation.
Vitamin D and arrhythmia
In a recent report, the correction of vitamin D deficiency and hypocalcemia resulted in control of incessant ventricular tachycardia and cardiomyopathy. A rare case of fetal atrial flutter was reported in vitamin D–resistant rickets. In an animal study, rats fed a vitamin D–deficient diet for 12 weeks developed significant QT-interval shortening despite normal serum calcium levels compared to normal rats. These findings suggest a possible role for vitamin D deficiency as a causal factor for arrhythmia and the need for further exploration.
Vitamin D and mortality
Numerous studies and meta-analyses suggest that vitamin D deficiency has a negative association with survival, while supplementation decreases overall mortality. A recently announced prospective randomized study plans to enroll 20,000 elderly subjects and examine the potential health benefits of vitamin D and omega-3 supplementation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


