ROLE OF PERINATAL CIRCULATORY PHYSIOLOGY ON CLINICAL MANIFESTATIONS AND MANAGEMENT OF NEONATAL HEART DISEASE
Introduction
In Chapter 1, the course of fetal circulation, mechanisms that sustain fetal circulatory pathways, allocation of the cardiac output to different organs, fetal cardiocirculatory regulation, the influence of cardiac defects on fetal circulation, and circulatory changes at birth were discussed. In this chapter, the role of postnatal circulatory adjustments on the clinical presentation and course of major congenital cardiac defects (CHDs) as well as their therapeutic implications will be reviewed.
CHD is usually endured well throughout fetal life. However, circulatory changes after birth have discernible effects on the clinical presentation and course of the cardiac disease, particularly at the beginning of the neonatal period.
Ductus Venosus (DV)
The DV is interposed between the umbilical vein (UV) and the inferior vena cava (IVC). A substantial proportion of umbilical venous blood goes through the DV in the fetus, thus carrying placental blood to the IVC. It is generally acknowledged that the DV is kept patent by the mechanical effect of the blood flow through it. After birth, the DV closes spontaneously, presumably due to lack of flow through it. However, the mechanism of closure may be similar to that of the ductus arteriosus (DA), as discussed in Chapter 1.
Total Anomalous Pulmonary Venous Connection (TAPVC)
In some forms of infradiaphragmatic type of TAPVC, pulmonary venous blood flow may be going through the DV. After birth, the DV closes spontaneously, resulting in greater pulmonary venous obstruction because it forces all the pulmonary venous blood to pass through the liver sinusoids. This obstruction is due to high impedance to the passage of blood flow through the hepatic circulation. The infant develops pulmonary edema and becomes symptomatic. In theory, prostaglandin E1 (PGE1) may open the DV, similar to the effect on the DA, but this favorable effect is not clearly documented.
Catheter Passage
Some pediatric cardiologists use the UV to perform cardiac catheterization and catheter interventional procedures, particularly in the very early neonatal period.1,2 This will allow placement of good-sized sheaths and avoid the use of percutaneous femoral vein entry. Once the DV closes, the umbilical venous route for catheter entry becomes impossible.
DA
The DA is a vascular channel that connects the pulmonary artery (PA) to the descending aorta. In the fetus, the DA redirects the deoxygenated blood from the PA into the descending aorta and from there, via the umbilical arteries, into the placenta for exchange of gases and excretion of metabolic waste products. During fetal life, the DA is kept patent by circulating and locally produced PGs. Subsequent to birth, the DA constricts and seals spontaneously; this closure is thought to be related to elevated PO2 as well as to progressively diminishing sensitivity of the ductal muscle to PGs with aging. The significance of the DA in diverse CHDs will be reviewed.
Hypoplastic Left Heart Syndrome (HLHS)
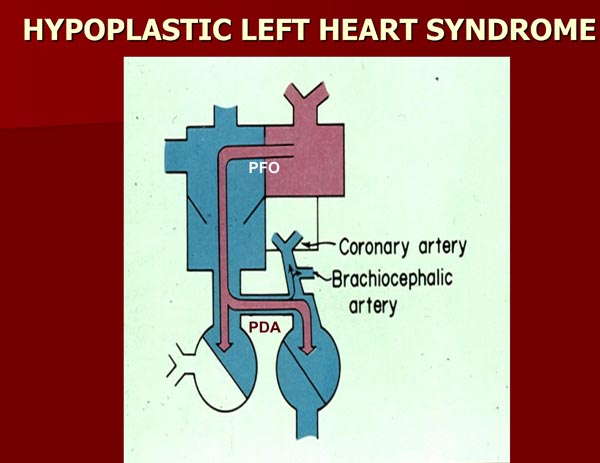
In HLHS, the mitral valve (MV), the left ventricle (LV), and/or the ascending aorta are markedly stenotic or atretic without forward flow from the left heart into the body.3–5 The whole systemic flow is dependent on the shunting of the blood from the PA into the descending aorta via the DA (Figure 2.1). After birth, the PO2 increases and the DA constricts, compromising the systemic blood flow. At the same time, a decline in the pulmonary vascular resistance (PVR) occurs, again due in part to an increase in PO2. Reduced PVR causes a marked increase in blood flow into the lungs. These events, especially when combined, produce decreased systemic perfusion with resultant severe acidemia, although the increased pulmonary-to-systemic flow ratio may augment arterial PO2. If not treated, severe congestive heart failure (CHF) and death will ensue.
Figure 2.1. Box diagram of HLHS. The pulmonary venous return cannot empty into the LV and therefore it has to egress into the right atrium via the PFO. If the FO is obstructive, the infant will develop signs of pulmonary venous obstruction. Since there is no forward flow from the left heart into the aorta, the systemic perfusion is reliant upon the patent ductus arteriosus (PDA). Backward flow into the brachiocephalic vessels and coronary arteries is also shown. If the ductus constricts, the systemic perfusion is likely to be compromised. Source: Reproduced with permission from Neonatology Today 2008;3(3):1–10.
Consequently, it is important to recognize that an increase in PO2 by rising ambient O2 concentration may not only hasten closure of the DA but also produce a fall in PVR. Intravenous infusion of PGE1 will aid in maintaining the ductus patent, thus preserving systemic perfusion. To promote systemic perfusion via the DA, the PVR should also be increased and the flow into the lungs decreased. Slightly higher PCO2 by mild hypoventilation and lower FIO2 than room air (15%–19%) may be used to increase pulmonary vasoconstriction and maintain adequate systemic perfusion while waiting for surgery.
Pulmonary Atresia with an Intact Ventricular Septum
Pulmonary atresia with an intact ventricular septum is a complex cyanotic CHD portrayed by total blockage of the pulmonary valve (PV), 2 separate ventricles, an open tricuspid valve (TV), and no ventricular septal defect (VSD).6 The right ventricle (RV) is frequently, but not always, small and hypoplastic. Because the PV is completely obstructed, the pulmonary blood flow is totally dependent on the patency of the DA (Figure 2.2). At birth, the DA is open and the pulmonary blood flow may be sufficient to maintain a good level of arterial O2 saturation. But, as the natural process of its closure ensues, the DA begins to constrict with resultant hypoxemia and metabolic acidosis.
Figure 2.2. Box diagram of hypoplastic right heart syndrome. Because there is no forward flow into the PA from the right ventricle, the pulmonary flow is dependent on the PDA. As the ductus begins to constrict, the pulmonary blood flow will decrease, causing severe hypoxemia. Source: Reproduced with permission from Neonatology Today 2008;3(3):1–10.
Table 2.1.Ductal-dependent pulmonary flow
| 1. Pulmonary atresia with an intact ventricular septum |
| 2. Critical pulmonary stenosis (also with an intact ventricular septum) |
| 3. Pulmonary atresia with a VSD |
| 4. Severe TOF |
| 5. Tricuspid atresia with severe pulmonary stenosis or atresia |
| 6. Any other complex cyanotic heart disease with severe pulmonary stenosis or pulmonary atresia |
| 7. Ebstein’s anomaly of the TV |
| 8. Hypoplastic right ventricle |
TAPVC
In TAPVC, all pulmonary veins eventually drain into the right atrium or systemic veins. In the infradiaphragmatic type of TAPVC, pulmonary venous obstruction is likely to be present. This results in a striking increase in the pulmonary arterial pressures and resistance and pulmonary edema. If the DA is patent, the pulmonary arterial tree may be decompressed, causing some relief of suprasystemic pulmonary pressures, although this is at the expense of deoxygenated blood bypassing the pulmonary circuit. Administration of intravenous PGE1 may be helpful in reducing PA pressures and supplementing cardiac output while waiting for emergent surgical intervention.
Transposition of the Great Arteries (TGA)
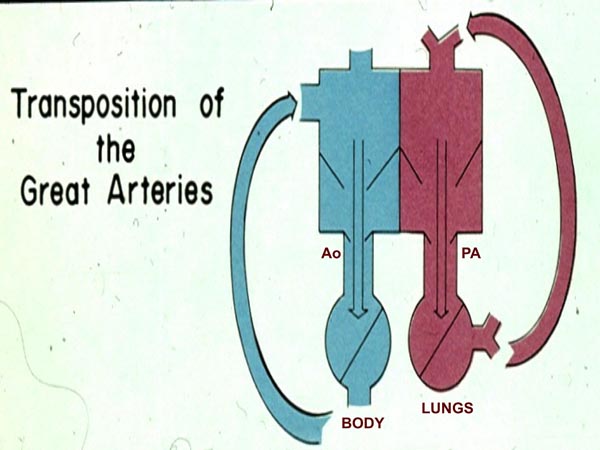
In TGA, the aorta comes off the RV and the PA from the LV. As a result, the pulmonary and systemic circulations are parallel (Figure 2.3) instead of the normal in-series circuits. Unless there is intercirculatory mixing across a patent foramen ovale (PFO) or DA, the baby is unlikely to stay alive because there is no delivery of oxygenated blood to the body and deoxygenated blood to the lungs. A patent DA may improve intercirculatory mixing with a resultant increase in arterial O2 saturation. Infusion of PGE1 is the first step in the management and is useful in lessening hypoxemia in some infants with TGA.
Figure 2.3. Box diagram of the heart in TGA. The right ventricle pumps the blood into the aorta (Ao) because of TGA which then goes to the body and returns into the right atrium and back into the body. Similarly, left ventricular blood goes to the PA and lungs and returns back to the left atrium and LV to be pumped back into the lungs. Thus the circulations are parallel instead of normal in-series circulation. Unless there are intercirculatory communications via either a PFO or a PDA, the infant cannot survive. Mixing across a VSD, if present (not shown in the diagram), would also prevent progressive hypoxemia and death. Source: Reproduced with permission from Neonatology Today 2008;3(3):1–10.
Aortic Coarctation
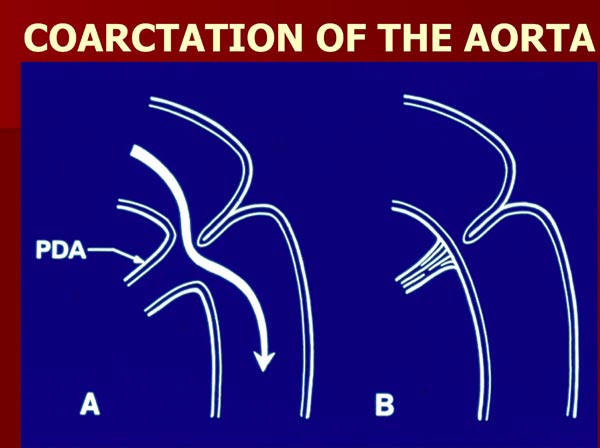
Formation of aortic coarctation is at least partly caused by a posterior shelf-like structure of the descending aorta with infolding of the media and intima of the aortic wall and partly as a result of closure of the DA.7,8 The flow of blood from the proximal part of the aorta into the descending aorta around the posterior shelf of coarctation is made possible when the DA is patent (Figure 2.4A). When the DA closes as a component of the natural process, this alternate path is not available any longer (Figure 2.4B). Aortic obstruction develops acutely with subsequent development of symptoms. Relief of obstruction may be achieved temporarily by infusion of PGE1.
Figure 2.4. Diagrammatic portrayal of aortic coarctation. (A) When the ductus (PDA) is patent, the flow from the aortic isthmus to the descending aorta in coarctation of the aorta bypasses the coarcted segment (A). When the PDA is closed (B), the flow is obstructed. Source: Reproduced with permission from Neonatology Today 2008;3(3):1–10.
Interrupted Aortic Arch (IAA)
This is an infrequent defect with a total lack of connection between the ascending and the descending aorta; this interruption may take place at different sites.9 Irrespective of the site of interruption, perfusion of the lower part of the body by blood flow from the PA to the descending aorta is preserved if the DA is patent. Again, during the normal course of development, the DA has a propensity to close, causing a lack of flow into the descending aorta. Infusion of PGE1 opens the DA and restores perfusion to the lower part of the body.
Critical Aortic Stenosis
In babies with critical aortic stenosis, the left ventricular output may be extremely poor. In such a situation, the open ductus supplements systemic output, preventing severe heart failure and metabolic acidosis. Administration of PGE1 to open the ductus may help to stabilize the baby while arrangements for transcatheter or surgical opening of the aortic valve are being made.
Foramen Ovale (FO)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree