The selection of patients with hypertrophic cardiomyopathy (HC) for the primary prevention of sudden death (SD) with implantable cardioverter-defibrillators (ICDs) has been determined by the assessment of 5 risk factors. We examined one of these markers, the family history of HC-related SD in first-degree relatives, for which few data are available. The rate of appropriate ICD interventions was assessed in 177 consecutive patients with HC (63% men, age 45 ± 14 years) who had undergone prophylactic implantation at 2 tertiary centers, according to the identification of ≥1 risk markers. During a follow-up period of 4.6 ± 3 years, 25 patients (14%) had experienced appropriate ICD interventions for ventricular tachycardia/fibrillation. The patients with a risk profile that included a family history of SD experienced interventions at a similar rate (3.7/100 person-years) as the patients without a family history of SD (3.1/100 person-years, p = 0.2). The rate and frequency of appropriate ICD interventions in 42 patients who had undergone implantation solely because of a family history of SD was 2.2/100 person-years (4/42, 10%), similar to that for patients with one risk factor other than SD family history (3.4%/100 person-years; 7/50, 14%; p = 0.2) and patients with multiple risk factors with (4.5/100 person-years; 9/49, 18%) and without (3.5/100 person-years; 5/36, 14%) a family history of SD (p = 0.8). In conclusion, a family history of SD is an important risk marker in patients with HC. Patients receiving ICDs for primary prevention because of a family history of HC-related SD, whether as an isolated risk factor or combined with other markers, experienced rates of appropriate ICD discharge comparable to that of other patient subsets with increased risk.
Hypertrophic cardiomyopathy (HC) is the most common cardiovascular cause of sudden death (SD) in the young, including competitive athletes. Implantable cardioverter-defibrillators (ICDs) are the only effective treatment option for prevention of SD due to ventricular tachyarrhythmias and have altered the natural history of this disease for many patients with HC. However, the identification of the subset of patients with HC at the greatest risk of SD can be challenging, given the heterogeneity of disease expression, the low and unpredictable event rate, and the relative infrequency of HC in the general cardiovascular practice. The selection of patients for the primary prevention of SD has been predicated on the assessment of several noninvasive risk factors. For one of these markers, a family history of HC-related SD, few data are available. Therefore, in the present cohort of patients with HC who had undergone ICD implantation, we systematically assessed the family history of SD as a risk factor, either alone or associated with other conventionally used clinical markers, to ascertain its value in identifying those patients at greatest risk of SD.
Methods
A total of 192 patients with HC and an ICD, evaluated at either the Mayo Clinic (n = 118) or the Minneapolis Heart Institute Foundation (n = 74), were enrolled in the present study. The SD risk profile was assessed at the first clinical evaluation at the 2 participating centers. Of the 192 patients, 15 had received an ICD for secondary prevention and were excluded from additional analysis. The final study cohort consisted of 177 patients (111 men [63%]; age 45.3 ± 14 years) with one or more major primary prevention SD risk factors, including (1) a family history of HC-related SD in ≥1 first-degree relatives (i.e., sudden, unexpected collapse in relatives who were previously in a stable clinical condition, with either a confirmed clinical/autopsy diagnosis of HC or SD at age <50 years without a definitive clinical or autopsy diagnosis); (2) a history of recent, unexplained syncope; (3) an abnormal blood pressure response during a treadmill exercise test (decrease, or failure to increase the systolic blood pressure >20 mm Hg); (4) massive left ventricular (LV) hypertrophy (maximum wall thickness ≥30 mm); or (5) one or more runs (≥3 beats) of nonsustained ventricular tachycardia at a rate of ≥120 beats/min on 24-hour ambulatory Holter electrocardiogram. Each of the 177 patients had an unequivocal diagnosis of HC as determined by 2-dimensional echocardiographic evidence of a hypertrophied and nondilated left ventricle in the absence of another cardiac or systemic disease that could account for the magnitude of hypertrophy at some time in their clinical course.
The decisions regarding risk status and ICD implantation were made according to customary practice by the managing cardiovascular specialists using established risk stratification markers for the primary prevention of SD. The follow-up duration was computed from the date of device implantation to the first appropriate ICD discharge. Stored intracardiac electrograms were analyzed to classify the arrhythmias responsible for precipitating the defibrillator discharges, according to the accepted definitions. Defibrillator discharges (shocks or antitachycardia pacing) were considered appropriate when triggered by ventricular fibrillation or rapid ventricular tachycardia (rate >200 beats/min) documented by the stored electrographic or cycle length data. Discharges were considered inappropriate when triggered by heart rates exceeding the programmed threshold, because of supraventricular arrhythmias or sinus tachycardia, or device malfunction documented by ICD interrogation.
Statistical analysis was performed using JMP, version 7.01, software (SAS Institute, Cary, North Carolina) to test for differences between the subgroups using analysis of variance for continuous variables and Fisher’s exact test for proportions. The event-rate per 100 person-years was calculated as the number of events divided by the sum of the follow-up years for the complete subgroup; 95% confidence intervals (CIs) were calculated using the modified Wald method. Survival was calculated using the Kaplan-Meier method and tested in the subgroups using the log-rank for trend. Data are presented as the cumulative event rates.
Results
The 177 study patients with HC and primary prevention ICDs were 45.3 ± 14 years old (range 16 to 80) at implantation, and 111 (63%) were men. The maximum LV wall thickness was 23 ± 8 mm (range 11 to 50), and a LV outflow tract gradient of ≥30 mm Hg at rest was present in 43 patients (24%). Eleven patients (6%) had progressed to the end-stage phase with systolic dysfunction (ejection fraction <50%). The demographic data are summarized in Table 1 .
| Variable | Total (n = 177) | Family History of SD (n = 91) | No Family History of SD (n = 86) | Risk Factor | |||
|---|---|---|---|---|---|---|---|
| Only Family History of SD (n = 42) | One Other Than Family History of SD (n = 50) | Multiple, Including Family History of SD (n = 49) | Multiple, Without Family History of SD (n = 36) | ||||
| Gender | |||||||
| Men | 111 | 49 | 62 | 22 | 35 | 27 | 27 |
| Women | 66 | 42 | 24 ⁎ | 20 | 15 | 22 | 9 |
| Age (years) | |||||||
| Mean | 45.3 ± 14 | 44.2 ± 14 | 46.5 ± 15 | 41.9 ± 12 | 45.9 ± 14 | 46.2 ± 15 | 47.3 ± 16 |
| Range | 16–80 | 16–80 | 16–78 | 17–60 | 17–69 | 16–78 | 16–80 |
| Follow-up (years) | 4.6 ± 3 | 4.7 ± 3 | 4.5 ± 3 | 4.5 ± 3 | 4.7 ± 3 | 4.8 ± 3 | 4.3 ± 3 |
| New York Heart Association class ≥3 | 52 (29%) | 26 (29%) | 26 (30%) | 12 (29%) | 18 (36%) | 14 (29%) | 8 (22%) |
| Left ventricular wall thickness (mm) | |||||||
| Mean | 23 ± 8 | 21 ± 7 | 24 ± 9 ⁎ | 21 ± 6 | 23 ± 8 | 22 ± 7 | 26 ± 9 ⁎ |
| Range | 11–50 | 11–55 | 12–43 | 12–32 | 11–51 | 12–43 | 13–55 |
| Left ventricular outflow tract gradient ≥30 mm Hg at rest | 43 (24%) | 20 (22%) | 23 (27%) | 10 (24%) | 14 (28%) | 10 (20%) | 9 (25%) |
| Ejection fraction | 66 ± 10% | 66 ± 10% | 66 ± 11% | 67 ± 9% | 66 ± 10% | 66 ± 11% | 64 ± 11% |
| End-stage hypertrophic cardiomyopathy (ejection fraction <50%) | 11 (6%) | 6 (7%) | 5 (6%) | 1 (2%) | 2 (4%) | 5 (10%) | 3 (8%) |
| Surgical myectomy | 51 (29%) | 25 (27%) | 26 (30%) | 12 (29%) | 20 (40%) | 13 (27%) | 6 (17%) |
| Medications at baseline | |||||||
| Amiodarone | 15 (9%) | 7 (8%) | 8 (9%) | 1 (2%) | 5 (10%) | 6 (12%) | 3 (8%) |
| β Blocker | 120 (68%) | 58 (64%) | 62 (72%) | 24 (57%) | 33 (66%) | 34 (70%) | 29 (81%) |
| Calcium blocker | 36 (20%) | 18 (20%) | 18 (21%) | 11 (26%) | 11 (22%) | 7 (14%) | 7 (20%) |
| Sodium blocker | 12 (7%) | 7 (8%) | 5 (6%) | 2 (5%) | 3 (6%) | 5 (10%) | 2 (6%) |
| Risk factors | |||||||
| Family history of sudden death | 91 (51%) | 91 (100%) | 0 | 42 (100%) | 0 | 49 (100%) | 0 |
| Unexplained syncope | 76 (43%) | 28 (31%) | 48 (56%) ⁎ | 0 | 18 (36%) | 28 (57%) | 30 (83%) ⁎ |
| Nonsustained ventricular tachycardia (on Holter monitor) | 64 (36%) | 20 (22%) | 44 (51%) ⁎ | 0 | 19 (38%) | 20 (41%) | 25 (69%) ⁎ |
| Left ventricular wall thickness ≥30 mm | 24 (14%) | 7 (14%) | 17 (20%) ⁎ | 0 | 7 (14%) | 7 (14%) | 10 (28%) |
| Abnormal blood pressure response to exercise | 20 (11%) | 7 (8%) | 13 (15%) | 0 | 6 (7%) | 7 (14%) | 7 (19%) |
Of the 177 patients, 91 (51%) had a family history of HC-related SD as a part of their risk profile. Of these 91 patients, a family history of SD was one of multiple SD risk factors present in 49 and was the sole risk factor identified for 42. The 91 patients with a family history of SD had a lower maximal LV wall thickness (21 ± 7 mm) than the 86 patients without a family history of SD (25 ± 9 mm; p = 0.04). After excluding patients with extreme hypertrophy (≥30 mm), no difference was found in the LV wall thickness between the patients with and without a SD family history (20.4 ± 5 vs 21.2 ± 5 mm, respectively; p = 0.3). Furthermore, no differences in the baseline demographics, medications, or other echocardiographic variables were observed between the patients with single versus multiple SD risk factors ( Table 1 ).
During follow-up, 25 (14%) of the 177 patients received an appropriate ICD intervention at 41 ± 14 years old. Most of these patients had received one appropriate ICD discharge (n = 14; 56%), whereas 11 had received more than one discharge on separate occasions (6 patients with 2 events, 3 with 3 events, and 2 with 4 events). The interval from implantation to the first appropriate ICD discharge was 2.7 ± 3 years (range 10 days to 8 years; Table 2 ).
| Variable | Total (n = 177) | Family History of SD (n = 91) | No Family History of SD (n = 86) | Risk Factor | |||
|---|---|---|---|---|---|---|---|
| Only Family History of SD (n = 42) | One Other Than Family History of SD (n = 50) | Multiple, Including Family History of SD (n = 49) | Multiple, Without Family History of SD (n = 36) | ||||
| Age at implant (years) | 39.4 ± 14 | 38.3 ± 14 | 40.6 ± 15 | 36.1 ± 12 | 40 ± 14 | 40.2 ± 15 | 41.5 ± 16 |
| Average number of risk factors assessed | 4.4 ± 0.6 | 4.3 ± 0.6 | 4.5 ± 0.6 | 4.3 ± 0.6 | 4.4 ± 0.6 | 4.4 ± 0.7 | 4.5 ± 0.5 |
| Patients with ≥4 risk factors assessed | 164 (93%) | 82 (90%) | 82 (95%) | 38 (90%) | 46 (92%) | 44 (90%) | 36 (100%) |
| Appropriate implantable cardioverter-defibrillator intervention | 25 (14%) | 13 (14%) | 12 (14%) | 4 (10%) | 7 (14%) | 9 (19%) | 5 (14%) |
| Interval to first implantable cardioverter-defibrillator intervention (years) | 3.1 ± 3 | 3.6 ± 3 | 1.9 ± 2 | 5.9 ± 2 | 1.7 ± 1 ⁎ | 2.6 ± 3 | 2.1 ± 2 |
| Age at first appropriate intervention (years) | 41.6 ± 14 | 41.2 ± 14 | 41.9 ± 15 | 33.1 ± 17 | 39.9 ± 13 | 44.8 ± 18 | 44.8 ± 12 |
| Rate of appropriate implantable cardioverter-defibrillator intervention/100 person-years | 3.4 (2.3–5.0) | 3.7 (2.1–6.3) | 3.1 (1.8–5.5) | 2.2 (0.6–5.6) | 3.4 (1.5–6.9) | 4.5 (2.3–8.5) | 3.5 (1.3–8.1) |
| Inappropriate implantable cardioverter-defibrillator shocks | 47 (27%) | 22 (26%) | 25 (27%) | 10 (24%) | 10 (20%) | 15 (31%) | 12 (33%) |
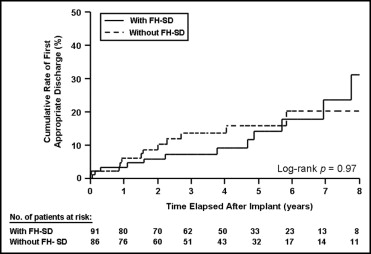
Of the 91 patients whose risk profile included a family history of SD, 13 (14%) had had an appropriate ICD intervention compared to 12 (14%) of the 86 patients without a family history of SD, but with other risk factors (p = 1.0; Table 2 ). The patients with a risk profile that included a family history of SD experienced appropriate ICD interventions at a rate of 3.7/100 person-years, similar to that of patients without a family history of SD (3.1/100 person-years; p = 0.2; Figure 1 ). Of the 11 patients with end-stage disease, 2 had had appropriate ICD interventions (both with multiple risk factors).
Of the 92 patients for whom the decision to pursue ICD placement was predicated on the presence of a single risk factor, 42 decisions (46%) were made solely on the presence of a family history of SD. For the remaining 50 patients (54%), the decision to implant the ICD was made because of unexplained syncope in 18, nonsustained ventricular tachycardia in 19, massive left ventricular hypertrophy in 7, and abnormal blood pressure response to exercise in 6 ( Table 1 ).
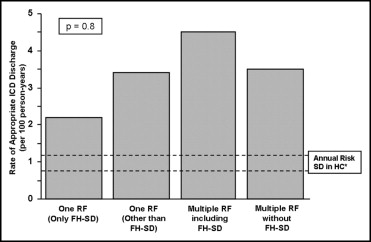
Of the 42 patients with an ICD placed solely because of a family history of SD, 4 (10%) experienced appropriate ICD discharges, with a cumulative event rate of 2.2/100 person-years (95% CI 0.6 to 5.6; Figure 2 ). Similarly, 7 of 50 patients (14%) with an ICD placed because of a single risk factor other than a SD family history had appropriate ICD discharges, with an event rate of 3.4/100 person-years (95% CI 1.5 to 6.9; p = 0.2; Figures 2 and 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


