34 Role of Adjunct Devices
Cutting Balloon, Laser, Ultrasound, and Atherectomy
The past 25 years have seen several mechanical approaches that ablate or section atheromatous plaque during percutaneous coronary intervention (PCI) to optimize acute results and reduce re-stenosis. Despite promising results from hundreds of small mechanistic studies, dozens of large randomized trials have failed to achieve predefined clinical and angiographic outcomes, and thus the routine use of atheroablative methods during PCI has not found support.1 In specific circumstances, however, the use of atheroablative devices has been beneficial and in selected cases the only means of achieving procedural and clinical success. This chapter analyzes the results of clinical trials and illustrates the use of adjunctive atheroablative therapies in contemporary practice.
 Historical Background
Historical Background
Before the modern era of coronary stenting, the search for treatments to overcome the limitations of PTCA was based on experimental studies, which showed that the healing response of treated coronary arteries was directly proportional to the degree of imposed injury.2 This was supported by angiographic analyses, which suggested that the degree of late re-stenosis was directly proportional to the gain achieved acutely during treatment and that the proportion between late loss and acute gain was consistent for a broad range of interventional devices.3 The decades-long search for a mechanical approach to excise or section atheromatous plaque emerged from the concept that plaque excision would improve clinical outcomes and lower the rate of re-stenosis after coronary intervention. Directional coronary atherectomy (DCA) entered clinical trials in 1987. Excimer laser coronary angioplasty (ELCA), percutaneous transluminal rotational atherectomy (PTRA), and transluminal extraction coronary atherectomy (TEC) were introduced in 1988. Holmium laser angioplasty (HLA) premiered in 1990, and cutting balloon angioplasty (CBA) debuted in 1991. Although each device used a different mechanism for modifying thrombus or atheromatous plaque, the common goal was to obtain larger acute gains and lower re-stenosis rates than could be achieved with PTCA. Evidence from randomized trials (Table 34-1),1,4–30 however, challenged the hypothesis that routine atheroablation during PCI is beneficial. The introduction of coronary stenting, particularly the use of drug-eluting stents (DESs; Chapter 13), rapidly replaced atheroablative therapies. Although atheroablative therapies may facilitate stent delivery and enhance stent expansion, the development of lower-profile, trackable, high-pressure balloon catheters (Chapter 15) has made PTCA the default method for lesion preparation before and after coronary stenting, and in many cases no lesion preparation is required at all before stent implantation.31–33
 Cutting Balloon Angioplasty
Cutting Balloon Angioplasty
Mechanism of Action
Compared with conventional PTCA, CBA makes controlled microincisions in the atheromatous plaque at lower pressures to reduce barotrauma. In an effort to overcome hoop stress, conventional PTCA stretches and dissects vascular tissue. After the balloon is deflated, the artery undergoes elastic recoil. Small mechanistic studies have suggested that lesions can be dilated at lower pressures with cutting balloon catheters than with conventional PTCA.21 In one study of 180 lesions, lumen enlargement was achieved at lower balloon pressures after CBA than after PTCA, and the increase in the cross-sectional area of plaque plus media measured by intravascular ultrasound (IVUS) was larger after CBA.34 In calcified lesions, CBA achieved larger lumen gain than did PTCA.
Equipment
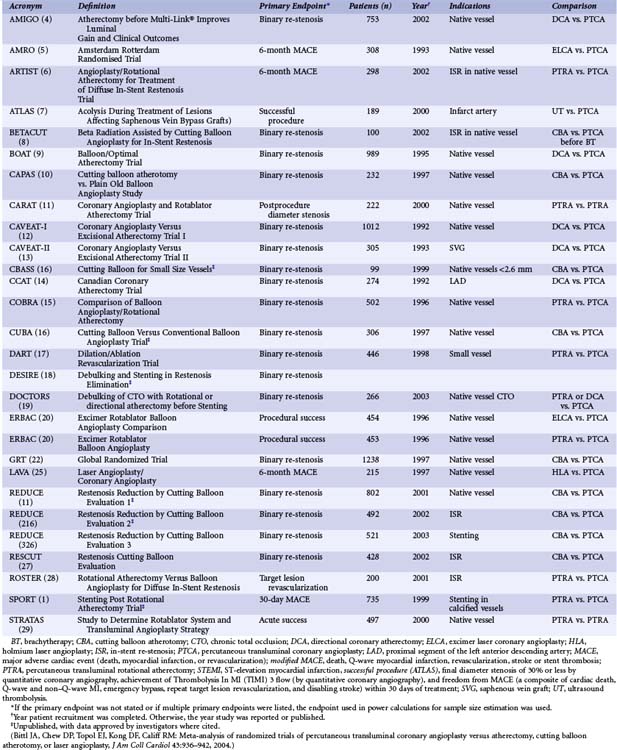
The Cutting Balloon Ultra-2™ is a monorail device, and the Flextome™ Cutting Balloon (Boston Scientific, Natick, MA) has two catheter designs (Fig. 34-1). The Flextome device contains a flex point every 5 mm along the length of the atherotomes (cutting blades) for greater flexibility and deliverability. It is available in over-the-wire and monorail configurations. Cutting balloons are available in balloon lengths of 6 mm, 10 mm, and 15 mm. The atherotomes are mounted longitudinally along the balloon surface. They are not directly affixed to the balloon but bonded to a pad mounted on the balloon. The double bond allows flexibility and ensures that the atherotomes remain firmly fixed in place. The number of atherotomes is determined by balloon diameter. Three atherotomes are on 2-mm and 3.25-mm balloons, and four are on 3.5-mm and 4-mm balloons.
Technique
The guidewires, catheters, and techniques used for cutting balloon angioplasty are similar to those for conventional PTCA (Chapter 16). However, cutting balloon catheters are less compliant and do not track as well as conventional balloon catheters. CBA may not be feasible when the proximal anatomy is tortuous. The risk of blade fracture or retention may be minimized by slowly inflating and deflating the balloon and by avoiding balloon pressures at or above the rated burst pressures.35
Clinical Results
Several small but largely positive trials of CBA, all involving less than 200 patients, reported that the use of CBA reduced re-stenosis by 41% to 69% compared with PTCA (Fig. 34-2).10,16,21,23,24 Other small studies evaluated CBA as pretreatment before brachytherapy for in-stent re-stenosis (ISR) and found no difference in re-stenosis rates between CBA and rotational atherectomy or between CBA and PTCA.8,30 Several large trials of CBA have been carried out (see Fig. 34-2). The GRT22 randomized 1238 patients and reported no difference in angiographic re-stenosis between CBA (31.4%) and PTCA (30.4%). The RESCUT study enrolled 428 patients with ISR and reported no difference in re-stenosis between CBA (29.8%) and PTCA (31.2%).27 The REDUCE 1 study enrolled 802 patients and reported slightly higher re-stenosis rates with CBA than with PTCA (32.7% vs. 25.5%).1 The REDUCE 2 study enrolled 416 patients and also observed a trend toward higher re-stenosis rates (52.1% vs. 44.2%). The REDUCE 3 study randomized 453 patients undergoing coronary stenting and reported lower re-stenosis rates after the use of CBA than after PTCA (11.8% vs. 19.6%).16,26
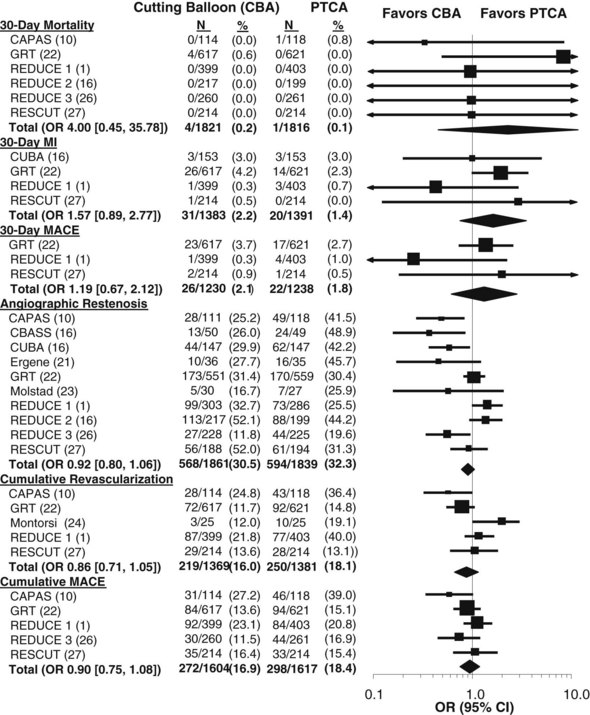
Figure 34-2 Systematic overview of randomized trials of cutting balloon angioplasty (CBA) versus percutaneous transluminal coronary angioplasty (PTCA). Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated to estimate the overall effect of CBA versus that of PTCA using an empirical Bayes model. Trial abbreviations are given in Table 34-1.
(Updated and reprinted from Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM: Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty, J Am Coll Cardiol 43:936–942, 2004. Copyright (2004), with permission from The American College of Cardiology Foundation.)
Lesion Selection
Several reports have suggested that CBA may be appropriate in small vessels bifurcation lesions or ostial stenoses.16,21 Bifurcation lesions have been a challenge for PTCA because of plaque shift and high re-stenosis rates, and CBA produced lower re-stenosis rates than PTCA (40% vs. 67%) in a small nonrandomized series of 87 patients with bifurcation lesions.36 Many interventional cardiologists use CBA for ostial lesions, but evidence of benefit for this indication has been elusive.37 The technical advantage of CBA is reduced slippage, especially for the treatment of ISR, which constitutes a common use of CBA. 22,26,27,37,38
Complications
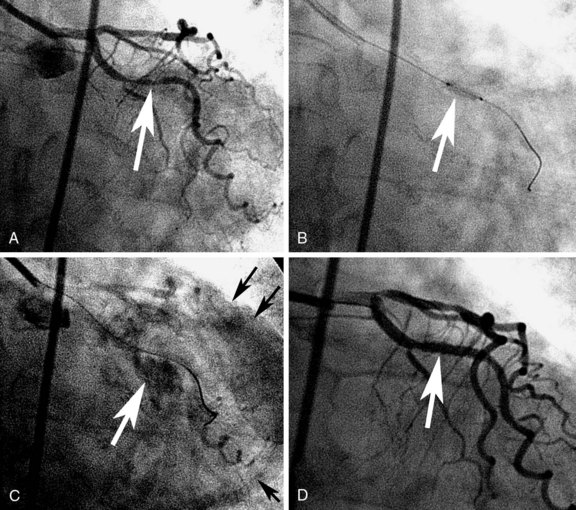
The risk of coronary perforation (Fig. 34-3) is slightly higher after the use of CBA than after conventional PTCA (0.8% vs. 0.0%), as reported in the GRT.22
 Ultrasound
Ultrasound
Clinical study
The ATLAS (Assessment of Treatment with Lisinopril And Survival) trial compared coronary ultrasound thrombolysis with abciximab treatment in 181 patients undergoing saphenous vein graft (SVG) PCI for acute coronary syndrome (ACS).7 The primary endpoint of a successful procedure and freedom from major adverse cardiac events (MACEs) was achieved in 63% of patients treated with ultrasound thrombolysis and in 82% of patients treated with abciximab (P = 0.008). The incidence of MACEs at 30 days was 25% with ultrasound thrombolysis and 12% with abciximab (P = 0.036). The use of ultrasound for native-vessel total occlusions is discussed in Chapter 24.