Indications/Contraindications
 Robotic-assisted pulmonary lobectomy may be considered for any patient undergoing lobectomy that does not involve complex vascular or airway reconstruction, or chest wall resection. The advantage of minimally invasive chest wall resection, which avoids rib spreading but still resects ribs is controversial. In our opinion we still most commonly favor thoracotomy when chest wall resection is required. Tumors larger than 7 cm (T3), tumors crossing fissures, and centrally located tumors may all be considered for robotic lobectomy with proper patient selection and increasing surgeon experience but in general these factors disfavor a robotic approach. Similarly, radiologic evidence of N1 nodes, induction chemotherapy and/or radiation, calcified lymph nodes, and prior thoracic surgery are not contraindications to robotic lobectomy but a robotic approach should not be selected early in one’s learning curve.
Robotic-assisted pulmonary lobectomy may be considered for any patient undergoing lobectomy that does not involve complex vascular or airway reconstruction, or chest wall resection. The advantage of minimally invasive chest wall resection, which avoids rib spreading but still resects ribs is controversial. In our opinion we still most commonly favor thoracotomy when chest wall resection is required. Tumors larger than 7 cm (T3), tumors crossing fissures, and centrally located tumors may all be considered for robotic lobectomy with proper patient selection and increasing surgeon experience but in general these factors disfavor a robotic approach. Similarly, radiologic evidence of N1 nodes, induction chemotherapy and/or radiation, calcified lymph nodes, and prior thoracic surgery are not contraindications to robotic lobectomy but a robotic approach should not be selected early in one’s learning curve.
 The typical contraindications for lobectomy that apply to patients undergoing resection via thoracotomy would also apply to patients undergoing robotic lobectomy (e.g., prohibitive lung function or medical comorbidities, multistation N2, gross N2 disease, or evidence of N3 disease). Patients with Pancoast tumors, tumors with extensive invasion into the mediastinum or esophagus, and contraindications to general anesthesia or single-lung ventilation are also poor candidates for robotic lobectomy. In addition, small nodules that are not tissue diagnosed that require lung palpation for wedge resection are considered by some as a contraindication for robotic lobectomy when a completely portal technique is used because of the inability to palpate the lung but lung palpation is possible with a robotic technique when a robotic-assisted technique is used. However, we have used navigational bronchoscopy with methylene blue tattooing of the nodules to help guide wedge resection or a robotic lymph node dissection and then conversion to VATS.
The typical contraindications for lobectomy that apply to patients undergoing resection via thoracotomy would also apply to patients undergoing robotic lobectomy (e.g., prohibitive lung function or medical comorbidities, multistation N2, gross N2 disease, or evidence of N3 disease). Patients with Pancoast tumors, tumors with extensive invasion into the mediastinum or esophagus, and contraindications to general anesthesia or single-lung ventilation are also poor candidates for robotic lobectomy. In addition, small nodules that are not tissue diagnosed that require lung palpation for wedge resection are considered by some as a contraindication for robotic lobectomy when a completely portal technique is used because of the inability to palpate the lung but lung palpation is possible with a robotic technique when a robotic-assisted technique is used. However, we have used navigational bronchoscopy with methylene blue tattooing of the nodules to help guide wedge resection or a robotic lymph node dissection and then conversion to VATS.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
 Preoperative evaluation including pulmonary function testing should be obtained. We routinely obtain stress testing to assess for myocardial ischemia especially in patients who have had a significant smoking history. Complete patient-specific staging should also be performed prior to lung resection. This includes PET-CT scan in most patients and the selective use of brain MRI or CT (those who are symptomatic or who have large central adenocarcinomas), endobronchial ultrasound-guided fine-needle aspiration (EBUS-FNA), esophageal ultrasound-guided fine-needle aspiration (EUS-FNA) for biopsy of the posterior-inferior lymph nodes and adrenals, and/or mediastinoscopy depending on the tumor size and institutional experience.
Preoperative evaluation including pulmonary function testing should be obtained. We routinely obtain stress testing to assess for myocardial ischemia especially in patients who have had a significant smoking history. Complete patient-specific staging should also be performed prior to lung resection. This includes PET-CT scan in most patients and the selective use of brain MRI or CT (those who are symptomatic or who have large central adenocarcinomas), endobronchial ultrasound-guided fine-needle aspiration (EBUS-FNA), esophageal ultrasound-guided fine-needle aspiration (EUS-FNA) for biopsy of the posterior-inferior lymph nodes and adrenals, and/or mediastinoscopy depending on the tumor size and institutional experience.
 When robotic techniques are used special considerations for robotic proficiency are needed as we have previously described—(add my reference of teaching robotics). These include documented scores of 70% or higher on simulator exercises, certificate of robotic safety training and cockpit awareness, weekly access to the robot, training of the entire personnel including the bedside assistant and familiarity with the robotic and the instruments, and a mandatory mastery of the pulmonary artery from both an anterior and posterior approach.
When robotic techniques are used special considerations for robotic proficiency are needed as we have previously described—(add my reference of teaching robotics). These include documented scores of 70% or higher on simulator exercises, certificate of robotic safety training and cockpit awareness, weekly access to the robot, training of the entire personnel including the bedside assistant and familiarity with the robotic and the instruments, and a mandatory mastery of the pulmonary artery from both an anterior and posterior approach.
 SURGERY
SURGERY
 As with any operation, planning each stage of the operation is crucial to ensure success. This begins with operating room setup when a robot is used. The robot adds anxiety to inexperienced robotic surgeons and anesthesiologists. Thus, planning of the room layout prior to the operation is critical. This includes the positioning of the bedside cart, the robot, the nurses’ table, the monitors, and the patient relative to the anesthesia equipment as needed. Because the robot is driven in over the patient’s head during lobectomy, the need for two monitors and the distance between the operating surgeon at the console and the scrub nurse and surgical assistant(s) who stand at the patient’s bedside, careful planning and communication is also needed.
As with any operation, planning each stage of the operation is crucial to ensure success. This begins with operating room setup when a robot is used. The robot adds anxiety to inexperienced robotic surgeons and anesthesiologists. Thus, planning of the room layout prior to the operation is critical. This includes the positioning of the bedside cart, the robot, the nurses’ table, the monitors, and the patient relative to the anesthesia equipment as needed. Because the robot is driven in over the patient’s head during lobectomy, the need for two monitors and the distance between the operating surgeon at the console and the scrub nurse and surgical assistant(s) who stand at the patient’s bedside, careful planning and communication is also needed.
Certain concepts specific to operating with robotic assistance should be mentioned here:
 The insertion of robotic instruments deserves special attention as does the passing of vascular staplers around fragile structure such as the pulmonary artery and/or vein. Carefully orchestrated moves and clear communication is needed between the bedside assistant and the surgeon. We have developed our own communication system between the bedside assistant and the surgeon to prevent iatrogenic injuries. This uses the anvil of the stapler as the hour hand of a clock and the degree of articulation is also quantified and communicated.
The insertion of robotic instruments deserves special attention as does the passing of vascular staplers around fragile structure such as the pulmonary artery and/or vein. Carefully orchestrated moves and clear communication is needed between the bedside assistant and the surgeon. We have developed our own communication system between the bedside assistant and the surgeon to prevent iatrogenic injuries. This uses the anvil of the stapler as the hour hand of a clock and the degree of articulation is also quantified and communicated.
 Robotic instruments should be initially inserted under direct vision during thoracic surgery for their initial placement. Once safely positioned instruments can then be quickly and safely inserted or changed for other instruments by properly using the memory feature of the robot that automatically inserts any new instruments to a position that is exactly 1 cm proximal to its latest position. However, if this feature is used it is incumbent on the surgeon to ensure that no vital structure has moved into the path of that newly placed instrument. The most common structure would be the lung.
Robotic instruments should be initially inserted under direct vision during thoracic surgery for their initial placement. Once safely positioned instruments can then be quickly and safely inserted or changed for other instruments by properly using the memory feature of the robot that automatically inserts any new instruments to a position that is exactly 1 cm proximal to its latest position. However, if this feature is used it is incumbent on the surgeon to ensure that no vital structure has moved into the path of that newly placed instrument. The most common structure would be the lung.
Operating Room Configuration
 One possible universal room setup employed for all types of robotic surgery including pulmonary resection is shown below in Figure 20.1.
One possible universal room setup employed for all types of robotic surgery including pulmonary resection is shown below in Figure 20.1.
 Consoles: The surgeon console should be positioned, so that good communication with the patient-side team can be established. The da Vinci surgical system console (Intuitive Surgical; Sunnyvale, CA) contains a microphone that amplifies the voice of the surgeon to the rest of the team. The presence of a second console permits easy exchange of control between surgeon, medical student, resident, or fellow for training purposes; this second console, if used, should be located fairly close to the primary console.
Consoles: The surgeon console should be positioned, so that good communication with the patient-side team can be established. The da Vinci surgical system console (Intuitive Surgical; Sunnyvale, CA) contains a microphone that amplifies the voice of the surgeon to the rest of the team. The presence of a second console permits easy exchange of control between surgeon, medical student, resident, or fellow for training purposes; this second console, if used, should be located fairly close to the primary console.
 Robot/bed: The approach of the robot to the patient’s side should be clear of any obstacles. The robot is driven over the patient’s head on a 15-degree angle to open up robotic arm 3 over their head and shoulder as shown in Figure 20.5A.
Robot/bed: The approach of the robot to the patient’s side should be clear of any obstacles. The robot is driven over the patient’s head on a 15-degree angle to open up robotic arm 3 over their head and shoulder as shown in Figure 20.5A.
 In addition, monitors are positioned for a clear view by both the bedside assistants and the scrub nurse.
In addition, monitors are positioned for a clear view by both the bedside assistants and the scrub nurse.
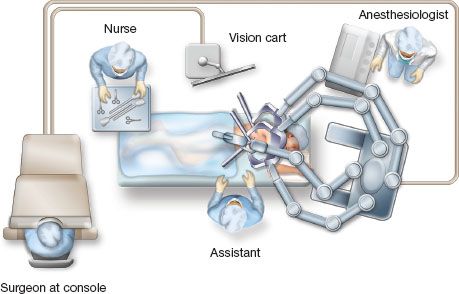
Figure 20.1 Operating room configuration for robotic lobectomy.
Depending on the size of the room and the arrangement of immobile structures within it, the patient’s bed may need to be turned such that the patient’s head is located well away from the ventilator and anesthesia console. A long extension for the endotracheal tubing should be used if this is necessary.
As the robot is set up before driving it in over the patient’s head, robotic arm 3 should be placed on the robotic side opposite to the side of the lobectomy (e.g., if performing a right-sided lobectomy, robotic arm 3 should be located on the robot’s left when facing it).
 Assistant: The assistant will be positioned on the patient’s ventral side (i.e., in front of the patient’s abdomen/chest), with a monitor opposite to them.
Assistant: The assistant will be positioned on the patient’s ventral side (i.e., in front of the patient’s abdomen/chest), with a monitor opposite to them.
 Scrub nurse: The scrub nurse will be positioned with their Mayo stand near or over the patient’s feet, as in conventional thoracotomy or VATS.
Scrub nurse: The scrub nurse will be positioned with their Mayo stand near or over the patient’s feet, as in conventional thoracotomy or VATS.
Patient Positioning
 General anesthesia is induced and the patient is intubated with a left-sided double-lumen endotracheal tube while supine. Proper placement of the double-lumen tube is assisted greatly by the use of a flexible pediatric bronchoscope and is critical to a smooth operation because access to the patient’s head and endotracheal tube will be limited by their positioning and the presence of the robot after docking.
General anesthesia is induced and the patient is intubated with a left-sided double-lumen endotracheal tube while supine. Proper placement of the double-lumen tube is assisted greatly by the use of a flexible pediatric bronchoscope and is critical to a smooth operation because access to the patient’s head and endotracheal tube will be limited by their positioning and the presence of the robot after docking.
 After the double-lumen tube is secured, the patient is positioned in lateral decubitus with the operative side up. Images of patient positioning are shown in Figure 20.2. An axillary roll is placed. We do not use an arm board, but rather place the patient with their back at the edge of the table, leaving space in front of their face to fold their arms and taking care to expose the axilla for port placement. We have used this positioning for over 17 years for our thoracotomies, but it is critical when using a four-arm robotic approach because it allows robotic arm 3 to move on a plane that is below the bed and avoid conflicts with that arm and the operative bed itself. Padding should be used around the arms and head to prevent nerve damage during the case. We use large foam pads to protect that patient’s head and arms.
After the double-lumen tube is secured, the patient is positioned in lateral decubitus with the operative side up. Images of patient positioning are shown in Figure 20.2. An axillary roll is placed. We do not use an arm board, but rather place the patient with their back at the edge of the table, leaving space in front of their face to fold their arms and taking care to expose the axilla for port placement. We have used this positioning for over 17 years for our thoracotomies, but it is critical when using a four-arm robotic approach because it allows robotic arm 3 to move on a plane that is below the bed and avoid conflicts with that arm and the operative bed itself. Padding should be used around the arms and head to prevent nerve damage during the case. We use large foam pads to protect that patient’s head and arms.
 This technique is easy, quick, and cheap; requires no special equipment and is reproducible. We position patients in under 10 minutes. The foam pad also helps protect the back of the patient’s head from link two of robotic arm 3. Tape should be used to secure the patient’s hips and upper body above the shoulder. The patient should be located with their flank (i.e., space between subcostal margin and iliac crest) directly over the break point of the bed, and the table should be flexed to increase the space between the ribs. A body warmer is applied to the lower body.
This technique is easy, quick, and cheap; requires no special equipment and is reproducible. We position patients in under 10 minutes. The foam pad also helps protect the back of the patient’s head from link two of robotic arm 3. Tape should be used to secure the patient’s hips and upper body above the shoulder. The patient should be located with their flank (i.e., space between subcostal margin and iliac crest) directly over the break point of the bed, and the table should be flexed to increase the space between the ribs. A body warmer is applied to the lower body.
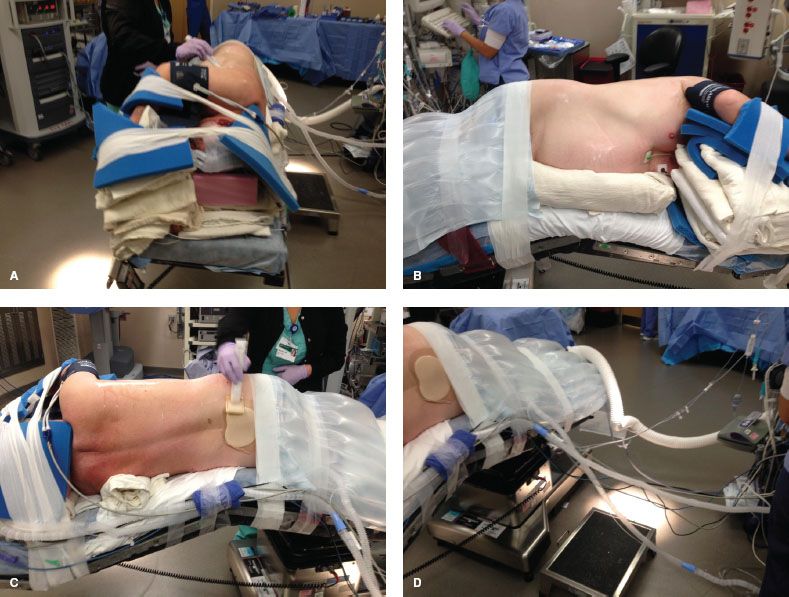
Figure 20.2 A: Patient positioning for robotic lobectomy. Foam pads for protection of pressure points of the head and arms are also shown. B: Patient positioning for robotic lobectomy. The axilla is exposed widely and the space between the patient’s iliac crest and costal margin is located above the break in the bed. C: Patient positioning for robotic lobectomy. The position of the axillary roll is shown. D: Patient positioning for robotic lobectomy, showing the distance between the anesthesia ventilator and the patient. Long flexible tubing is used to facilitate this and is taped along the bed with the other monitoring lines to provide easier access to the posterior aspect of the patient if a thoracotomy becomes necessary.
Port Placement/Docking
 The ports are all inserted in the seventh intercostal space, over top of the eighth rib, for upper/middle lobectomy, and in the eighth intercostal space, over top of the ninth rib for lower lobectomy.
The ports are all inserted in the seventh intercostal space, over top of the eighth rib, for upper/middle lobectomy, and in the eighth intercostal space, over top of the ninth rib for lower lobectomy.
 The ports are marked as follows: Robotic arm 3 (5-mm port) is located 1 to 2 cm lateral from the spinous process of the vertebral body, robotic arm 2 (8 mm) is 10 cm medial to robotic arm 3, the camera port (we prefer the 12-mm camera) is 9 cm medial to robotic arm 2, and robotic arm 1 (12 mm) is placed right above the diaphragm anteriorly. The assistant port (12 mm) is placed as low as possible in the chest, triangulated exactly halfway in between the most anterior robotic port (which is robotic arm 1 in the right chest and robotic arm 2 in the left chest) and the camera port and then as low as possible to remain just above the diaphragm, which is being pushed downward by the insufflating humidified CO2 gas (Fig. 20.3).
The ports are marked as follows: Robotic arm 3 (5-mm port) is located 1 to 2 cm lateral from the spinous process of the vertebral body, robotic arm 2 (8 mm) is 10 cm medial to robotic arm 3, the camera port (we prefer the 12-mm camera) is 9 cm medial to robotic arm 2, and robotic arm 1 (12 mm) is placed right above the diaphragm anteriorly. The assistant port (12 mm) is placed as low as possible in the chest, triangulated exactly halfway in between the most anterior robotic port (which is robotic arm 1 in the right chest and robotic arm 2 in the left chest) and the camera port and then as low as possible to remain just above the diaphragm, which is being pushed downward by the insufflating humidified CO2 gas (Fig. 20.3).
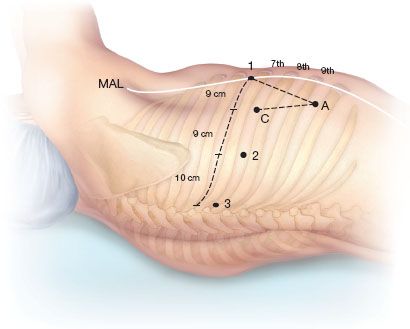
Figure 20.3 Port placement for right robotic lobectomy. C, camera port; 1, robotic arm 1; 2, robotic arm 2; 3, robotic arm 3; A, assistant port; MAL, midaxillary line.
Sequence of Port Placement
 A 5-mm port is placed first in the camera port position and CO2
A 5-mm port is placed first in the camera port position and CO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


