Clinical studies suggest an association between bisphosphonate use and new-onset atrial fibrillation (AF). Intravenous bisphosphonates more potently increase the release of inflammatory cytokines than do oral bisphosphonates; thus, the risk of developing AF may be greater with intravenous preparations. We have evaluated incidence of new-onset AF with use of oral and intravenous bisphosphonates through a systematic review and meta-analysis of the literature. We searched PubMed, CINAHL, Cochrane Central Register of Controlled Trials, Scopus, and EMBASE databases for observational studies and randomized controlled trials (RCTs) published from 1966 to April 2013 that reported the number of patients developing AF with use of oral or intravenous bisphosphonates. The random-effects Mantel-Haenszel test was used to evaluate the relative risk of AF with use of oral and intravenous bisphosphonates. Nine studies (5 RCTs and 4 observational studies) were included in the final analysis. Pooled data from RCTs and observational studies (n = 135,347) showed a statistically significantly increased risk of new-onset AF with both intravenous (relative risk 1.40, 95% confidence interval 1.32 to 1.49) and oral (relative risk 1.22, 95% confidence interval 1.14 to 1.31) bisphosphonates. The z statistic, which assesses the difference between the 2 risk ratios, indicated higher risk of AF with intravenous bisphosphonates versus oral bisphosphonates (p = 0.03). In conclusion, pooled data from RCTs and observational studies suggest that risk of AF is increased by use of oral or intravenous bisphosphonates but further suggest that risk is relatively greater with intravenous preparations.
Bisphosphonates typically are the first-line therapy for treatment of osteoporosis and osteopenia, conditions that affect >40 million Americans. Although bisphosphonates effectively reduce the risk of osteoporotic fractures, recent studies have identified several potential adverse effects, including atrial fibrillation (AF), raising safety concerns. Studies reporting increased risk of AF with bisphosphonates have included both oral and intravenous forms. It is possible that risk may differ depending on the route of administration (oral vs intravenous); the latter is associated with relatively more robust release of inflammatory cytokines, which might potentiate development of AF. Hence previous results may have been driven by intravenous bisphosphonate use alone. However, studies that have reported outcomes separately for oral and intravenous bisphosphonates have evidenced contradictory findings. Therefore, we performed a systematic review and meta-analysis of published reports to evaluate the risk of developing AF with oral or intravenous bisphosphonates.
Methods
Meta-analysis was performed in accordance with the Meta-analysis Of Observational Studies in Epidemiology guidelines. A checklist of each of the Meta-analysis Of Observational Studies in Epidemiology criteria and how they were addressed in our study is in Supplementary Table 1 .
PubMed, EMBASE, CINAHL, Scopus, the Web of Science, and Cochrane Central Register of Controlled Trials were searched for studies evaluating the safety and efficacy of oral and intravenous bisphosphonates that reported AF as an outcome. Search terms were bisphosphonate, aminobisphosphonate, AF, and arrhythmia. The search was last updated on September 30, 2013. Pertinent trials were also searched in clinicaltrials.gov and in the proceedings of major international cardiology meetings (American College of Cardiology, American Heart Association, European Society of Cardiology, and Transcatheter Cardiovascular Therapeutics). Two independent reviewers (AS and AV) performed study selection; divergences were resolved by consensus. Studies were included if they met all of the following criteria: (1) human studies with participants of any age requiring bisphosphonate therapy for any indication, (2) randomized controlled trials or observational studies with contemporaneous control groups, and (3) studies reporting the outcome of AF separately for oral and intravenous bisphosphonates. Two investigators extracted key study and patient characteristics and relevant outcomes, independently (AS and AV).
Mix 2.0 Pro (BiostatXL) software was used for analysis. A random-effects model with inverse variance weighting was used to calculate pooled relative risks (RRs; and 95% confidence interval [CI]) for developing AF with (1) oral bisphosphonates, (2) intravenous bisphosphonates, and (3) both oral and intravenous bisphosphonates. We also calculated the absolute risk and absolute risk difference of developing AF with oral and intravenous bisphosphonates. Furthermore, we calculated a z test for the difference between the 2 risk ratios by pooling the standard errors derived from the confidence limits for oral and intravenous bisphosphonates using Microsoft Excel 2007 (Microsoft, Redmond, Washington). Heterogeneity among studies was assessed using Cochran Q test and I 2 statistic, which denotes the percentage of total variation across studies that is a result of heterogeneity rather than chance. Heterogeneity was considered significant if the p value was <0.05. Publication bias was assessed with visual inspection of funnel plots and the regression test of Egger. The influence of individual studies was examined by removing each study individually, one at a time, to assess the degree to which the meta-analysis estimate was dependent on a particular study (exclusion sensitivity analysis).
Results
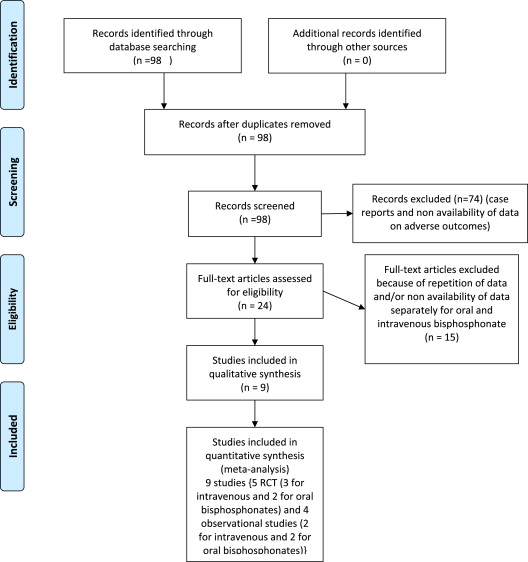
Our MEDLINE search returned 98 studies. After eliminating duplicate results, no registry returned additional studies. Of the 98 potentially evaluable studies, 74 were excluded because of nonavailability of data on adverse outcomes. The remaining 24 studies were assessed for satisfaction of the inclusion or exclusion criteria. Nine studies (5 randomized controlled trials [RCTs] and reports analyzing data from RCTs; 4 observational studies) met all criteria and were included in this analysis ( Figure 1 ; Tables 1 and 2 ). In these 9 studies, 5 reported the outcome of AF with intravenous bisphosphonates (3 RCTs and 2 observational studies) and the remaining 4 studies presented the risk of AF with oral bisphosphonates (2 RCTs and 2 observational studies). The study by Huang et al compared oral bisphosphonate with raloxifene. The remaining studies compared bisphosphonate with placebo.
| Characteristic | Black et al | Lyles et al | Cummings et al | Karam et al | Black et al |
|---|---|---|---|---|---|
| Bisphosphonates, n | 3,862 | 1,054 | 3,236 | 5,020 | 616 |
| Control, n | 3,852 | 1,057 | 3,223 | 5,048 | 617 |
| Study description | Randomized double-blind | Randomized double-blind | Retrospective analyses of FIT | Retrospective analyses of phase 3 clinical trials ∗ | Extension of HORIZON PFT for 3 more yrs |
| Type of bisphosphonate used | Intravenous zolendronic acid | Intravenous zolendronic acid | Oral alendronate | Oral risedronate | Intravenous zolendronic acid |
| Comparator | Placebo | Placebo | Placebo | Placebo | Placebo |
| Mean age (yrs) | 73 | 74 | 69 | 73 | 75 |
| Men (%) | 0 | 24 | 0 | 2 | 0 |
| Mean follow-up (mo) | 36 | 24 | 48 | 24 | 72 |
∗ Vertebral Efficacy with Risedronate Therapy Trial, the Bone Mineral Density Trial, the Risedronate 5 mg Daily Prevention Trial, the Glucocorticosteroid-Induced Osteoporosis Prevention and Treatment Trials, and the Hip Intervention Program trial.
| Characteristic | Abrahamsen et al | Erichsen et al | Huang et al | Wilkinson et al |
|---|---|---|---|---|
| Type of bisphosphonate used | Oral (alendronate, clodronate, etidronate, ibandronate, and risedronate) | Intravenous (zolendronate and pamidronate) | Oral (alendronate) | Intravenous (zolendronate and pamidronate) |
| Bisphosphonates, n | 14,302 | 3,981 | 21,037 | 6,857 |
| Control, n | 28,731 | 7,906 | 6,220 | 13,714 |
| Mean age (yrs) | 74 | 65 | 74 | ≥65 |
| Men (%) | 11 | 39 | 0 | 40 |
| Hypertension (%) | B: 51 | B: n/a | B: 53 | B: 52 |
| C: 48 | C: n/a | C: 55 | C: 53 | |
| Diabetes (%) | B: 5 | B: 6 | B: 21 | B: 13 |
| C: 6 | C: 5 | C: 24 | C: 14 | |
| Hyperlipidemia (%) | B: 7 | B: n/a | B: n/a | B: n/a |
| C: 7 | C: n/a | C: n/a | C: n/a | |
| COPD (%) | B: n/a | B: 6 | B: 22 | B: 3 |
| C: n/a | C: 7 | C: 23 | C: 2 | |
| Thyroid disorder (%) | B: n/a | B: 2 | B: 1 | B: 19 |
| C: n/a | C: 2 | C: 1 | C: 17 |
We used the published Meta-analysis Of Observational Studies in Epidemiology checklist to select the studies for this review ( Figure 1 ). We used the published standard criteria for reporting of randomized clinical trials and observational studies checklist to select the studies for this review. All observational studies were intermediate to low-intermediate bias risk as assessed by the Newcastle-Ottawa Scale for quality assessment risk evaluation of adequacy of selection, comparability, and outcomes assessment for individual studies ( Table 3 ).
| Source | Adequacy of Selection | Comparability | Outcomes Assessment | ||||
|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Nonexposed Cohort | Ascertainment of Exposure | Assessment of Outcomes | Follow-Up Period Long Enough for Outcome to Occur | Adequacy of Follow-Up Period Among Cohorts | ||
| Abrahamsen et al | ** | ** | ** | *** | ** | ** | ** |
| Erichsen et al | * | * | ** | *** | ** | ** | ** |
| Huang et al | ** | ** | ** | *** | ** | ** | ** |
| Wilkinson et al | * | * | ** | *** | ** | ** | ** |
∗ Studies demonstrate that outcome of interest was not present at the start of the study.
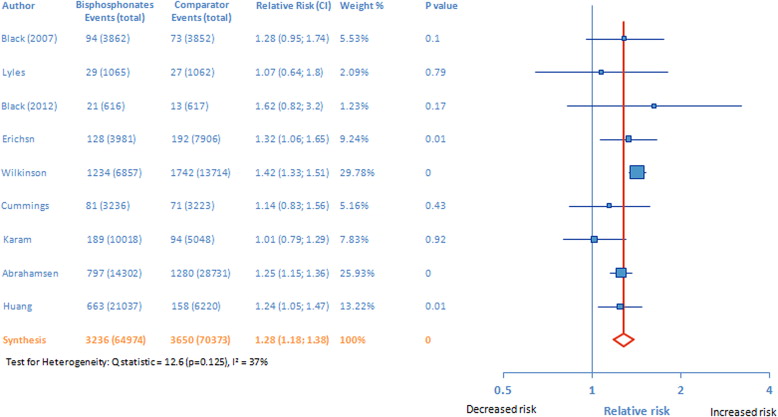
On pooling the results of all 9 studies, the risk of AF was increased with bisphosphonate therapy versus the nonbisphosphonate comparators (pooled RR 1.28, 95% CI 1.18 to 1.38; Figure 2 ). Next we examined the risk for AF with oral and intravenous bisphosphonates separately. As shown in Figure 3 , the risk of AF was higher for both intravenous (RR 1.40, 95% CI 1.32 to 1.49) and oral (pooled RR 1.22, 95% CI 1.14 to 1.31) bisphosphonates than for comparators. Absolute risks of developing AF with intravenous and oral bisphosphonates were 0.043 (vs 0.032 for the control arm) and 0.030 (vs 0.026 for the control arm), respectively. The absolute risk difference for developing AF was 0.011 (1.1%) and 0.0040 (0.4%) for intravenous and oral bisphosphonates, respectively. The z test for assessing the difference between 2 risk ratios indicated a greater risk of AF with intravenous versus oral bisphosphonates (p = 0.003, z statistic = 2.93); even by using the larger of the 2 standard errors (for intravenous bisphosphonates) instead of the pooled standard error, a p value of 0.047 was obtained.