Patients with left ventricular (LV) systolic dysfunction after myocardial infarction (MI) are at particularly high risk for recurrent adverse outcomes. The magnitude of the decrease in risk associated with smoking cessation after MI has not been well described in patients with LV dysfunction after MI. We aimed to quantify the risk decrease associated with smoking cessation in subjects with LV dysfunction after MI. The Survival and Ventricular Enlargement (SAVE) trial randomized 2,231 subjects with LV dysfunction 3 to 16 days after MI. Smoking status was assessed at randomization and at regular intervals during a median follow-up of 42 months. Propensity score-adjusted Cox proportional hazard models were used to quantify the decrease in risk of all-cause mortality, death or recurrent MI, and death or heart failure (HF) hospitalization associated with smoking cessation. In baseline smokers who survived to 6 months without interval events, smoking cessation at 6-month follow-up was associated with a significantly lower adjusted risk of all-cause mortality (hazard ratio [HR] 0.57, 95% confidence interval [CI] 0.31 to 0.91), death or recurrent MI (HR 0.68, 95% CI 0.47 to 0.99), and death or HF hospitalization (HR 0.65, 95% CI 0.46 to 0.92). In conclusion, in patients with LV dysfunction after MI, smoking cessation is associated with a 40% lower hazard of all-cause mortality and a 30% lower hazard of death or recurrent MI or death or HF hospitalization. These findings indicate that smoking cessation is beneficial after high-risk MI and highlight the importance of smoking cessation as a therapeutic target in patients with LV dysfunction after MI.
Studies of smoking cessation after myocardial infarction (MI) have consistently demonstrated significant decreases in risk of death or recurrent MI. A recent large meta-analysis of 20 prospective cohort studies estimated a 36% relative risk decrease in mortality and a 32% relative risk decrease in recurrent MI. However, the effect of smoking cessation in patients after MI complicated by left ventricular (LV) dysfunction has not been well characterized. We used data from stable survivors after MI complicated by LV dysfunction to quantify the risk decrease associated with smoking cessation after MI.
Methods
The Survival And Ventricular Enlargement (SAVE) trial was a randomized, double-blind, placebo-controlled trial of the efficacy and safety of captopril therapy after high-risk MI with evidence of LV systolic dysfunction (LV ejection fraction ≤40% by radionuclide ventriculography). Detailed protocol and results of this study have been previously published. Briefly, subjects were enrolled 3 to 16 days after MI. Major exclusion criteria included serum creatinine concentration >2.5 mg/dl, heart failure (HF) requiring vasodilator therapy, or intolerance or contraindication to an angiotensin-converting enzyme (ACE) inhibitor. The primary study outcome was mortality from any cause. Secondary end points included cardiovascular (CV) death, hospitalization for HF, severe HF requiring open-label ACE inhibitor therapy, recurrent MI, and a composite of these. From January 1987 to January 1990, 2,231 subjects were enrolled. Median follow-up was 42 months.
Smoking status was assessed at randomization, 2 weeks after randomization, every 3 months after randomization for the first year, and every 4 months up to 5 years after randomization. At randomization, investigators were asked whether the subject had ever smoked cigarettes and whether the subject had smoked within 3 weeks before hospitalization. At each subsequent visit, investigators were asked whether the subject had smoked since the previous visit. Smokers at baseline were defined as those who had reported smoking within 3 weeks of hospitalization for the index MI. Persistent smoking status after MI and quitters were defined based on reported smoking status at the 6-month follow-up visit. Intermittent smokers during the follow-up period were classified as smokers.
The primary outcomes of interest for this analysis were all-cause mortality, death or recurrent MI, and death or HF hospitalization during follow-up. All events were reported by the site investigator and independently adjudicated by an Endpoints Review Committee coordinated from the Brigham and Women’s Hospital, Boston, Massachusetts. Definitions of these end points have been previously published.
Continuous variables are presented as mean ± SD unless otherwise specified. Comparison of baseline characteristics between smokers and nonsmokers and between persistent smokers and quitters was performed with Fisher’s exact test for categorical variables and t test for continuous variables. Two-sided p values <0.05 were considered statistically significant. Sample size was allowed to float. Cumulative incidence of outcome events during follow-up is expressed as proportion and incidence is expressed as rates per 100 person-years of follow-up.
To quantify the risk decrease associated with smoking cessation in subjects who smoked at the time of index MI, we evaluated stable baseline smokers who survived to 6 months without an interval recurrent MI or HF hospitalization. Outcomes in subjects who continued smoking at the 6-month follow-up visit were compared to subjects who had stopped. Risk decrease associated with smoking cessation was assessed using Cox proportional hazard models. The number of outcome events for this analysis suggested that the model would support only 4 to 7 covariates. Therefore, a propensity score for smoking status at 6-month follow-up was generated using the 24 parameters from baseline and 6-month follow-up listed in Table 1 . Performance of the resulting logistic regression model for predicting smoking status at 6 months in baseline smokers was acceptable with a model c-statistic of 0.70. After adjustment for propensity score, no baseline parameter remained significantly associated with 6-month smoking status at a p value <0.72. All Cox proportional hazard models were adjusted for this propensity score.
| Variable | Continued Smoking | Stopped Smoking | p Value |
|---|---|---|---|
| (n = 268) | (n = 463) | ||
| Age (years) | 54.1 ± 10.7 | 54.5 ± 10.4 | 0.6243 |
| Women | 41 (15.3%) | 88 (19.0%) | 0.2275 |
| White | 239 (89.2%) | 419 (90.5%) | 0.6091 |
| College degree | 23 (8.6%) | 72 (15.6%) | 0.0084 |
| Medical history | |||
| Diabetes mellitus | 33 (12.3%) | 70 (15.1%) | 0.3218 |
| Heart failure | 15 (5.6%) | 11 (2.4%) | 0.0361 |
| Myocardial infarction | 97 (36.2%) | 102 (22.0%) | <0.0001 |
| Hypertension | 98 (36.6%) | 143 (30.9%) | 0.1214 |
| Baseline physical examination | |||
| Body mass index (kg/m 2 ) ⁎ | 25.8 (23.0–28.7) | 26.3 (23.5–29.6) | 0.0680 |
| Systolic blood pressure (mm Hg) | 111 ± 15 | 109 ± 13 | 0.0725 |
| Diastolic blood pressure (mm Hg) | 69 ± 9 | 69 ± 9 | 0.6903 |
| Heart rate (beats/min) | 77 ± 12 | 76 ± 12 | 0.3354 |
| Left ventricular ejection fraction (%) | 32.1 ± 6.6 | 31.6 ± 6.3 | 0.3702 |
| Killip class >I | 102 (38.1%) | 186 (40.2%) | 0.5834 |
| New York Heart Association class >I | 97 (36.2%) | 121 (26.1%) | 0.0056 |
| Baseline laboratory values | |||
| Glomerular filtration rate (ml/min/1.73m 2 ) | 73.4 ± 22.1 | 76.1 ± 20.7 | 0.1078 |
| Creatinine kinase (times above upper limit of normal) ⁎ | 10.1 (4.7–18.0) | 12.6 (6.5–21.5) | 0.0011 |
| Active treatment group | 145 (54.1%) | 228 (49.2%) | 0.2196 |
| Intervention between index myocardial infarction and randomization | |||
| Catheterization | 157 (59.0%) | 274 (59.7%) | 0.8755 |
| Cardiac surgery | 18 (6.8%) | 44 (9.6%) | 0.2162 |
| Percutaneous transluminal coronary angioplasty | 48 (18.1%) | 94 (20.5%) | 0.4393 |
| Thrombolytic therapy | 90 (33.7%) | 185 (40.4%) | 0.0810 |
| Clinical status at 6-month follow-up | |||
| Angina pectoris | 66 (24.7%) | 92 (19.9%) | 0.1358 |
| Clinical heart failure | 9 (3.4%) | 39 (8.4%) | 0.0079 |
To assess the durability of the effect of smoking cessation, we also determined the risk decrease in subsequent CV outcomes associated with smoking cessation for 12, 16, and 24 months after MI. We performed a sensitivity analysis to assess the potential impact of exposure misclassification due to changes in smoking status during follow-up. Because the increase in CV risk associated with smoking has been shown to largely dissipate by 2 years after smoking cessation, this sensitivity analysis was performed by modeling smoking status as a time-varying covariate with a 2-year lag.
Results
Out of 2,231 subjects enrolled in SAVE, baseline smoking status was available in 2,230. Nine hundred twenty-four (41.4%) smoked within 3 weeks before hospitalization and 1,306 (58.6%) did not ( Figure 1 ). Of these nonsmokers at baseline, 963 (73.7%) were former smokers and 343 (26.3%) had never smoked. Compared to nonsmokers, smokers were younger (55.0 ± 10.7 vs 62.5 ± 9.3 years), had fewer medical co-morbidities including diabetes (16% vs 26%), hypertension (34% vs 47%), and previous MI (31% vs 39%), and had lower body mass index (26.0 vs 26.5 kg/m 2 ), blood pressure (systolic blood pressure 110 ± 14 vs 114 ± 15 mm Hg), and heart rate (77 ± 12 vs 78 ± 13), and better renal function (estimated glomerular filtration rate 74.5 ± 21.6 vs 66.8 ± 19.8 ml/min/1.73 m 2 ). Smokers tended to have larger infarcts as assessed by peak serum creatinine kinase level (expressed as times above upper limit of normal, 11.5 vs 9.0) and more frequently underwent catheterization (58% vs 53%) and received thrombolytic therapy (36% vs 31%).
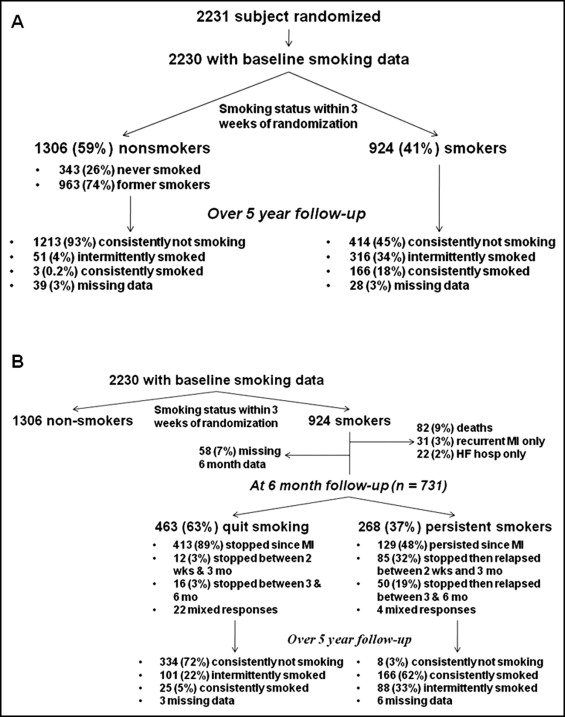
Of the 1,306 nonsmokers at baseline, the overwhelming majority (93%) consistently reported not smoking during follow-up ( Figure 1 ). However, in the 924 smokers at baseline, smoking status during follow-up was much more variable. One hundred sixty-six (18%) consistently reported smoking during follow-up, 316 (34%) reported smoking intermittently, and 414 (45%) consistently reported not smoking during the follow-up period.
To assess the magnitude of decrease in risk in baseline smokers associated with smoking cessation, we evaluated stable baseline smokers who survived to 6 months after MI without interval recurrent MI or HF hospitalization ( Figure 1 ). Of 924 baseline smokers, 82 (8.9%) died, 50 (5.4%) had a recurrent MI (19 of whom subsequently died, 38.0%), and 36 (3.9%) were hospitalized for HF (14 of whom subsequently died, 38.9%) within 6 months. Of the remaining 789 subjects, 58 (7%) had missing smoking status at 6 months.
Of the 731 subjects in this analysis, 268 (37%) were smokers at 6-month follow-up and 463 (63%) were not ( Figure 1 ). Of nonsmokers at 6 months, the large majority (89%) had stopped by the first follow-up visit after MI (at 2 weeks). Three hundred thirty-four (72%) reported consistently not smoking during the follow-up period, whereas 101 (22%) intermittently reported smoking and only 25 (5%) consistently reported smoking during follow-up. Of smokers at 6 months, 129 (48%) had persistently reported smoking since the baseline visit. One hundred thirty-five (50%) initially stopped smoking after MI but 85 (32%) relapsed between the 2-week and 3-month follow-up visit and 50 (19%) relapsed between the 3- and 6-month follow-up visit. Over the follow-up period after the 6-month visit, 166 (62%) consistently reported smoking, 88 (33%) intermittently reported smoking, and only 8 subjects (3%) consistently reported not smoking.
Baseline characteristics in subjects who stopped smoking at 6-month follow-up were generally similar to persistent smokers ( Table 1 ). Persistent smokers more frequently had previous HF and previous MI, had smaller infarcts based on peak creatinine kinase level, and less frequently had clinical HF symptoms at 6-month follow-up.
Unadjusted incidence and event rates of outcomes during the follow-up period are listed in Table 2 . Compared to quitters, persistent smokers had a higher incidence of all-cause mortality (16.4% vs 8.9%, event rate 5.9 vs 3.1 per 100 person-years), death or recurrent MI (22.8% vs 14.9%, event rate 8.6 vs 5.5 per 100 person-years), death or HF hospitalization (26.1% vs 18.6%, event rate 9.5 vs 6.6 per 100 person-years), and a composite of death, recurrent MI, HF hospitalization, or stroke (27.2% vs 20.4%, event rate 10.0 vs 7.3 per 100 person-years).
| Outcome | Continued Smoking (n = 268) | Stopped Smoking (n = 463) | ||||
|---|---|---|---|---|---|---|
| Events | Person-Years | Event Rate | Events | Person-Years | Event Rate | |
| Death | 44 (16.4%) | 742 | 5.9 | 41 (8.9%) | 1,308 | 3.1 |
| Recurrent myocardial infarction | 24 (9.0%) | 41 (8.9%) | ||||
| Heart failure hospitalization | 37 (13.8%) | 54 (11.7%) | ||||
| Stroke | 12 (4.5%) | 14 (3.1%) | ||||
| Death or recurrent myocardial infarction | 61 (22.8%) | 711 | 8.6 | 69 (14.9%) | 1,263 | 5.5 |
| Death or heart failure hospitalization | 70 (26.1%) | 739 | 9.5 | 86 (18.6%) | 1,303 | 6.6 |
| Death, recurrent myocardial infarction, heart failure hospitalization, or stroke | 72 (27.2%) | 722 | 10.0 | 93 (20.4%) | 1,266 | 7.3 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


