Recent studies have suggested that proton pump inhibitors (PPIs) might reduce the inhibitory effect of clopidogrel on platelet aggregation, possibly through inhibition of the hepatic cytochrome P450 2C19 (CYP2C19) isoenzyme. The prevalence of CYP2C19 loss-of-function alleles is much greater among East Asians than among other populations. Thus, potential drug interactions might be more apparent. Therefore, we conducted a nationwide, population-based study using the Taiwan National Health Insurance database. We identified 3,278 patients (mean age 65.9 ± 11.9 years, 71.9% men) with coronary artery disease who had taken clopidogrel after percutaneous coronary intervention from the 1 million sampling cohort data set since January 1, 2002. Of the 3,278 patients, 572 had received concomitant PPIs for underlying gastrointestinal disease and 2,706 had not used PPIs. To the end of 2007, 1,410 patients had been rehospitalized, 970 patients had undergone revascularization, and 499 patients had died. According to the Kaplan-Meier analysis, the incidence of rehospitalization (p = 0.001) and mortality (p <0.001) was significantly greater for the patients with concomitant PPI use than for those without concomitant PPI use. However, the incidence of revascularization was similar in the 2 groups. Multivariate analyses showed that concomitant PPI use was associated with an increased risk of rehospitalization (hazard ratio 1.23, 95% confidence interval 1.07 to 1.41, p = 0.003) and mortality (hazard ratio 1.65, 95% confidence interval 1.35 to 2.01, p <0.001). In conclusion, the concomitant use of clopidogrel and PPIs should be done with care to avoid adverse outcome in East Asians patients who have undergone percutaneous coronary intervention.
Clopidogrel, the platelet aggregation inhibitor, is metabolized through hepatic cytochrome P450 2C19 (CYP2C19), and is widely prescribed to patients with coronary artery disease (CAD). Proton pump inhibitor (PPI) use, which is indicated in patients with peptic ulcer disease, is also metabolized through CYP2C19. Polymorphism of the CYP2C19 has been classified into 3 genotype groups: the rapid extensive metabolizer group, the intermediate metabolizer group, and the poor metabolizer group. The average percentage of poor metabolizer subjects was 2% to 4.8% worldwide, except for a markedly greater incidence of up to 22.5% among East Asians. Potential drug interactions between clopidogrel and PPI use might be more apparent in East Asians. Currently, the results of co-administration of a PPI and clopidogrel have been inconsistent and conflicting. Therefore, we conducted a nationwide population-based study to investigate the possible influence of PPI use on clopidogrel use in patients with CAD undergoing percutaneous coronary intervention (PCI) using the Taiwan National Health Insurance database.
Methods
The National Health Insurance program in Taiwan has operated since 1995 and enrolls nearly all the inhabitants of Taiwan (21,869,478 beneficiaries of 22,520,776 inhabitants at the end of 2002). Currently, the National Health Insurance Research database (NHIRD) at the National Health Research Institutes (NHRI) (available at: www.nhri.org.tw/nhird/ ) in Miaoli (Taiwan) takes charge of the complete National Health Insurance claims database and has published several dozens of extracted data sets for researchers. The NHRI has released a cohort data set made of 1 million randomly sampled people who were alive during 2000 and collected all the records on these subjects from 1995 onward. It is also one the largest nationwide population-based databases in the world. These random samples have been confirmed by the NHRI to be representative of the Taiwanese population. In this cohort data set, a patient’s original identification number has been encrypted to protect privacy. However, the encrypting procedure has been consistent, so the linkage of claims belonging to the same patient is feasible within the NHIRD data sets. First, we identified the patients who underwent PCI by procedure code and case payment code from the 1 million sampling cohort data set since January 1, 2002. Next, we identified the patients who had used of clopidogrel after PCI. These patients were further divided into 2 groups according to whether they had concomitant use of PPI. The covariate variables included age, gender, and pre-existing (in the year before treatment) hypertension ( International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 401.xx to 405.xx), diabetes mellitus (250.xx), hyperlipidemia (272.x), ischemic stroke (434.x, 436, 437.1), and chronic renal disease (580.xx to 587.xx). The end points of the study included (1) rehospitalization for CAD, including acute myocardial infarction (the first 2 ICD-9-CM codes 410xx or 411xx) or angina (the first 2 ICD-9-CM codes 413xx or 414xx); (2) revascularization, including PCI or coronary artery bypass graft; or (3) mortality, which was identified by the date of in-hospital death or decreased of the national health insurance without additional claims data.
Microsoft SQL Server 2005 was used for data management and computing. A statistical analysis was performed using Statistical Package for Social Sciences software, version 15.0 (SPSS, Chicago, Illinois). All data are expressed as the frequency (percentage), mean ± SD, or median with the interquartile range. The parametric continuous data between the different groups were compared by an unpaired Student’s t test. The categorical data between the different groups were compared using a chi-square test and Yates’ correction or Fisher’s exact test, as appropriate. Survival analysis was assessed using a Kaplan-Meier analysis, with the significance determined using the log-rank test. The survival time was calculated from the date of PCI to the date of rehospitalization, revascularization, or mortality. A multiple regression analysis was performed using a Cox proportional hazard regression analysis. Statistical significance was inferred at a 2-sided p value of <0.05.
Results
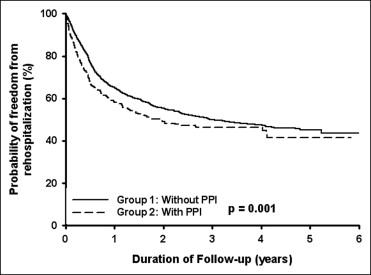
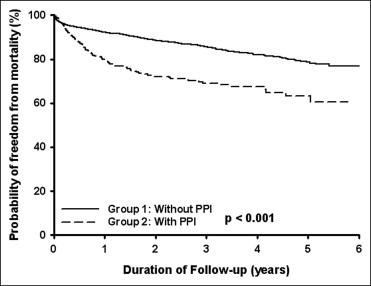
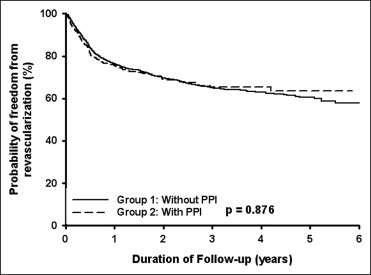
A total of 3,278 patients (mean age 65.9 ± 11.9 years, 71.9% men) had received clopidogrel after the first PCI procedure from January 2002 to December 2007. Of these patients, 572 had had concomitant PPI use for underlying gastrointestinal disease and 2,706 who had not had concomitant PPI use ( Table 1 ). The patients with concomitant PPI use were older and had more hypertension, diabetes mellitus, ischemic stroke, and chronic kidney disease but less hyperlipidemia than patients without concomitant PPI use. To the end of 2007, 1,410 patients (43.0%) had been rehospitalized (because of acute myocardial infarction or angina), 970 patients (29.6%) had undergone revascularization (including PCI and coronary artery bypass grafting), and 499 deaths (15.2%). According to the Kaplan-Meier analysis, the incidence of rehospitalization (p = 0.001; Figure 1 ) and the incidence of mortality (p <0.001; Figure 2 ) were significant greater in the patients with concomitant PPI use than in those without PPI use. However, the incidence of revascularization was similar in the 2 groups (p = 0.876; Figure 3 ). Multivariate analyses showed that concomitant PPI use (hazard ratio 1.23, 95% confidence interval 1.07 to 1.41, p = 0.003) and age, male gender, diabetes mellitus, hyperlipidemia, and ischemic stroke predicted rehospitalization after PCI ( Table 2 ). Multivariate analyses showed that only age, male gender, and hyperlipidemia predicted revascularization after PCI ( Table 3 ). Multivariate analyses showed that concomitant PPI use (hazard ratio 1.65, 95% confidence interval 1.35 to 2.01, p <0.001) and age, diabetes mellitus, hyperlipidemia, ischemic stroke, and chronic kidney disease predicted mortality after PCI ( Table 4 ).
| Variable | All (n = 3,278) | PPI Use | p Value | |
|---|---|---|---|---|
| Yes (n = 572) | No (n = 2,706) | |||
| Age (years) | 65.9 ± 11.9 | 68.7 ± 11.0 | 65.3 ± 12.0 | <0.001 ⁎ |
| Men | 2,358 (71.9%) | 408 (71.3%) | 1,950 (72.1%) | 0.723 |
| Hypertension | 2,942 (89.7%) | 542 (94.8%) | 2,400 (88.7%) | <0.001 ⁎ |
| Diabetes mellitus | 1,807 (55.1%) | 359 (62.8%) | 1,448 (53.5%) | <0.001 ⁎ |
| Hyperlipidemia | 2,500 (76.3%) | 412 (72.0%) | 2,088 (77.2%) | 0.009 ⁎ |
| Ischemic stroke | 881 (26.9%) | 179 (31.3%) | 702 (25.9%) | 0.009 ⁎ |
| Chronic kidney disease | 1,082 (33.0%) | 282 (49.3%) | 800 (29.6%) | <0.001 ⁎ |
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age (years) | 0.99 | 0.99–1.00 | 0.012 ⁎ |
| Male gender | 1.15 | 1.02–1.30 | 0.023 ⁎ |
| Hypertension | 1.17 | 0.96–1.43 | 0.118 ⁎ |
| Diabetes mellitus | 1.13 | 1.00–1.27 | 0.043 ⁎ |
| Hyperlipidemia | 1.32 | 1.16–1.52 | <0.001 ⁎ |
| Ischemic stroke | 1.14 | 1.01–1.28 | 0.034 ⁎ |
| Chronic kidney disease | 1.11 | 0.98–1.24 | 0.089 |
| Proton pump inhibitor use | 1.23 | 1.07–1.41 | 0.003 ⁎ |
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age (years) | 0.99 | 0.99–1.00 | 0.024 ⁎ |
| Male gender | 1.20 | 1.04–1.40 | 0.016 ⁎ |
| Hypertension | 1.25 | 0.98–1.59 | 0.074 |
| Diabetes mellitus | 1.12 | 0.97–1.28 | 0.124 |
| Hyperlipidemia | 1.48 | 1.24–1.75 | <0.001 ⁎ |
| Ischemic stroke | 1.00 | 0.86–1.16 | 0.987 |
| Chronic kidney disease | 1.06 | 0.92–1.22 | 0.449 |
| Proton pump inhibitor use | 1.02 | 0.86–1.21 | 0.825 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


