Chapter 24 Rigid Bronchoscopy with Y Stent Insertion at Left Secondary Carina
Case Description
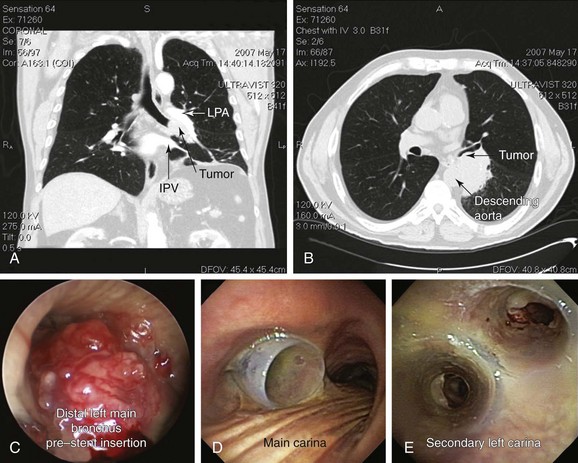
A 75-year-old man with a 120–pack-year history of smoking and COPD (FEV1 52% predicted, DLCO 39% predicted) developed progressive dyspnea, a weak and hoarse voice, and hemoptysis. His usual exertional dyspnea had progressed to the point that he now required assistance with activities of daily living. Chest CT showed a 6 × 5 × 7 cm left infrahilar mass involving the distal left main bronchus (LMB) and the entrance to the left upper and lower lobe bronchi (Figure 24-1, A and B). In addition, mediastinal lymphadenopathy was significant in stations 7 (subcarinal) and 4 L (left lower paratracheal). Flexible bronchoscopy showed an immobile left vocal cord and near complete obstruction in the distal LMB characterized by extrinsic compression and exophytic endoluminal tumor with hypervascular mucosa (Figure 24-1, C). Examination of the airways distal to the tumor was possible using forced saline lavage and showed patent distal segments but significant involvement of the left secondary carina (LC2). Rigid bronchoscopy was performed under general anesthesia with spontaneous assisted ventilation. Endobronchial needle aspiration (EBNA) with rapid on-site evaluation (ROSE) revealed non–small cell lung cancer (NSCLC). Nd:YAG laser photocoagulation and bronchoscopic debulking partially restored patency to left upper and lower lobe bronchi. However, tumor infiltration, cartilaginous collapse, and extrinsic compression required insertion of a large Y silicone stent (16 mm diameter tracheal limb, 12 mm bronchial limbs) placed within the left main bronchus (Figure 24-1, D and E).
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient required considerable assistance at home. His functional impairment was consistent with a Karnofsky Performance Scale score (KPS) of 50. At the time of hospitalization, however, KPS was only 20 (severely disabled, where hospitalization was necessary but death was not imminent).1 His Eastern Cooperative Oncology Group (ECOG) performance scale, which may be a better predictor of prognosis than KPS,2,3 was 3 (i.e., the patient was capable of only limited self-care and was confined to bed or chair for more than 50% of waking hours). Assessment of this patient’s performance status is essential for guiding therapeutic interventions because this degree of impairment, due to lung cancer or comorbid conditions (e.g., chronic obstructive pulmonary disease [COPD]), may preclude resection or, alternatively, chemoradiotherapy.4 In fact, this patient was not operable based on his pulmonary function tests and functional status. Relevant to his case, however, is that the combination of interventional bronchoscopy and external beam radiation therapy (EBRT) might improve survival, symptoms, and quality of life, as has been previously shown in patients with lung cancer, central airway obstruction, and KPS less than 50.
The patient’s weak voice and hoarseness are explained by unilateral (left) vocal cord immobility as noted on bronchoscopy (see video on ExpertConsult.com) (Video V.24.1![]() ). This was likely due to unilateral recurrent laryngeal nerve (RLN) injury, which caused the affected vocal fold to rest in the paramedian position. The contralateral vocal fold may or may not provide adequate glottic closure during phonation. If closure is not achieved, the residual glottal gap results in air escape with a weak voice secondary to a “leaky valve” phenomenon.5 In unilateral RLN injury, the glottic aperture is usually adequate, and related dyspnea and stridor are rare. Glottal incompetence, however, increases the risk for aspiration, particularly with thin liquids. Cough, which requires transient tight glottic closure, may also be affected; this is important in cases of airway stent insertion with risk for mucus plugging and difficulty raising secretions. Associated sensory loss from RLN involvement may also cause dysphagia, a symptom not seen in our patient. With regard to staging, RLN involvement is consistent with T4 disease. In conjunction with N2 disease on computed tomography (CT) scan (involvement of stations 7 and 4 L), this patient’s clinical stage is at least IIIB, and estimated median survival time is 10 months and 5 years for 7% despite multimodality treatment.6
). This was likely due to unilateral recurrent laryngeal nerve (RLN) injury, which caused the affected vocal fold to rest in the paramedian position. The contralateral vocal fold may or may not provide adequate glottic closure during phonation. If closure is not achieved, the residual glottal gap results in air escape with a weak voice secondary to a “leaky valve” phenomenon.5 In unilateral RLN injury, the glottic aperture is usually adequate, and related dyspnea and stridor are rare. Glottal incompetence, however, increases the risk for aspiration, particularly with thin liquids. Cough, which requires transient tight glottic closure, may also be affected; this is important in cases of airway stent insertion with risk for mucus plugging and difficulty raising secretions. Associated sensory loss from RLN involvement may also cause dysphagia, a symptom not seen in our patient. With regard to staging, RLN involvement is consistent with T4 disease. In conjunction with N2 disease on computed tomography (CT) scan (involvement of stations 7 and 4 L), this patient’s clinical stage is at least IIIB, and estimated median survival time is 10 months and 5 years for 7% despite multimodality treatment.6
Comorbidities
This patient had moderate, Global Obstructive Lung Disease (GOLD) stage II COPD. Although no other comorbidities were reported, careful examination and review of systems are warranted because COPD is associated with hypertension, coronary heart disease, stroke, obstructive sleep apnea, depression, anxiety, and cognitive dysfunction, which could preclude or complicate interventions under general anesthesia.4
Support System
The patient had good social support from his daughter and his wife. Early discussions about quality of life and symptom concerns are warranted because patients suffering from cancer will commonly develop pain, dyspnea, cough, and fatigue during the course of their illness.7 These conversations took place with the patient and his family because a diagnosis of cancer has a significant psychological and emotional impact on family members and caregivers as well.8
Patient Preferences and Expectations
This gentleman had expressed his desire for diagnosis and was ready to consider all available treatment options. He was very clear, however, that he did not want to live “being dependent on machines,” and he wanted to make all decisions regarding his care. In the United States, most patients diagnosed with cancer want a shared or active role in the decision-making process, usually with greater participation than what actually occurs. Role preferences (active vs. passive), however, vary greatly during decision making, and repeated assessments are required to meet patients’ expectations and improve their satisfaction with treatment decisions.9
Procedural Strategies
Indications
This patient required tissue diagnosis. We elected to proceed with rigid bronchoscopy under general anesthesia to restore airway patency with laser and silicone stent insertion and to diagnose the airway lesion using endobronchial biopsy, bronchial washing, and/or endobronchial needle aspiration. Chemoradiotherapy currently constitutes the standard of care for patients with inoperable locally advanced non–small cell lung cancer (NSCLC).10 This treatment, however, is usually restricted to patients who maintain a good performance status.11
Central airway obstruction (CAO) in the setting of NSCLC is associated with a very poor prognosis. In one study, median and 1 year survival of patients with malignant CAO were reportedly as low as 3.4 months and 15%, respectively.12 However, patients with advanced NSCLC with locally treated CAO combined with systemic chemotherapy might have survival rates similar to those patients without CAO treated with chemotherapy alone.13 Interventional bronchoscopic procedures in this patient with inoperable disease are expected to restore airway patency and improve lung function, dyspnea, and functional status, thus allowing initiation of systemic therapy, which could improve his survival.14
Contraindications
No absolute contraindications to rigid bronchoscopy were noted. Although COPD treatment should be optimized before elective interventions to reduce perioperative complications, our patient had stable disease at the time of evaluation and was being treated with short-acting β2-agonists and long-acting anticholinergic agents in accordance with international guidelines.15
Expected Results
For diagnosis, endobronchial needle aspiration (EBNA) with rapid on-site evaluation (ROSE) was performed. In patients with visible endobronchial lesions such as this, the diagnostic yield increases from 76% with conventional procedures alone to 96% with EBNA in addition to conventional procedures (washings, brushings, and biopsy).16
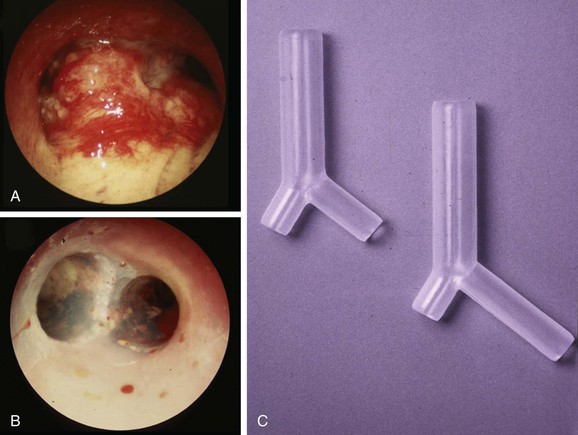
For restoring airway patency in our patient with near complete obstruction in the distal left main bronchus (LMB), left secondary carina (LC2) involvement, and a mixed pattern of obstruction (extrinsic and endoluminal exophytic), a straight metal or silicone stent would not have restored patency to the obstructed lobar bronchi. Therefore we decided to insert a silicone Y stent. This type of stent can be used for patients with fixed or dynamic CAO from benign or malignant disease, but the most common uses are to restore airway patency, to improve dyspnea and quality of life for patients with malignant CAO involving the mainstem bronchi and the lower trachea (Figure 24-2), and to reduce recurrent infection in patients with tracheoesophageal or bronchoesophageal fistulas.17–20 Y stents are also effective in restoring airway patency, and they improve symptoms in patients with severe diffuse tracheobronchomalacia, but with a high rate of nonfatal complications.21,22 In our patient, a Y silicone stent insertion was planned to restore and maintain LMB and left upper lobe (LUL) and left lower lobe (LLL) airway patency, which would allow the following:
1. Initiation of systemic therapy: By improving functional status, this patient could become a candidate for systemic treatment; in fact, evidence suggests that patients with advanced NSCLC and CAO who undergo successful interventional bronchoscopy to relieve airway obstruction might have survival similar to that of patients without CAO.13
2. Improved exercise capacity and dyspnea: A significant improvement in symptoms in up to 94% of patients undergoing central airway stent insertion has been reported, although 40% of patients required multiple procedures. This raises questions about the costs per quality of life-years in patients with advanced disease.14
Team Experience
Studies reporting an association between results of interventional bronchoscopy, procedure-related complications, and the required level of technical expertise have not yet been performed. It is known, however, that survival and complications after such procedures are worse for malignant than for benign CAO, and it was suggested that these patients should be considered distinct populations to be analyzed and reported separately.23 One can only hypothesize that referral to an experienced bronchoscopist and oncologic team will result in reduced complications, more rapid diagnosis, greater restoration of airway patency, and earlier discharge from the hospital.
Risk-Benefit Analysis
For diagnosis, we chose to perform EBNA before proceeding with laser photocoagulation. In our experience, EBNA has a lower risk of bleeding and provides immediate diagnosis when ROSE is available. In contrast with endobronchial biopsy, however, tissue architecture is not seen.16 With regard to restoring airway patency, the risks of further physiologic compromise and massive bleeding were considered to be outweighed by the benefit of restoring airway patency, the ability to diagnose the lesion, and improved functional status and the offer of systemic therapy. Alternatives to our approach should be considered on a case-by-case basis.
Therapeutic Alternatives for Restoring Airway Patency
These include endobronchial and systemic therapies.
• Endobronchial brachytherapy (EBB): This technique has proven efficacy in patients with endoluminal tumor and a substantial extrabronchial component. It is based on the principle of inverse square law, which states that dose rate decreases as a function of the inverse square of the distance to the source center, making it possible to achieve a high irradiation dose in the center of the irradiation source with a fast decrease toward the periphery. EBB offers significant palliation with rates of recanalization ranging from 60% to 90%, and symptomatic improvement is seen in 70% to 80% of patients. The variability in reported results is explained by patient selection, different treatment schemata, and the use of additional treatments. For palliation of NSCLC symptoms, however, a Cochrane meta-analysis concluded that EBB alone was less effective than EBRT. For patients previously treated by EBRT who are symptomatic from recurrent CAO, however, EBB may be considered.24 EBB is usually performed via flexible bronchoscopy. Effects are delayed, and complications include hemoptysis, which could be fatal in up to 21% of patients, as well as fistula formation, radiation bronchitis (10%), and bronchial stenosis. This modality was not considered optimal for our patient because of his severe airway obstruction and marginal pulmonary function. Furthermore, EBB for this tumor would have to be extensive, including the LMB along with the LUL and the LLL. Treatment in the upper lobes is associated with the highest incidence of hemoptysis, probably because of the proximity of great vessels.
• Photodynamic therapy (PDT): This treatment can be performed via flexible bronchoscopy and is approved for local regional palliation for advanced NSCLC. This modality is most effective when more than 50% narrowing results from mucosal disease.25 The outcome of PDT seems to be best, however, when patients have a relatively good performance status.26 In addition, associated risks of phototoxicity for approximately 4 weeks post intervention (≈20%) and a therapeutic effect that is delayed for at least 48 hours make it less than ideal in our patient. PDT might actually worsen airway obstruction during the initial post-treatment period because of sloughing of airway mucosa and retained tumor debris. Similar to EBB, PDT is contraindicated in patients at high risk for fatal massive hemoptysis.
• Cryotherapy: This approach causes thrombosis and necrosis of tumor tissues and could have been used for the exophytic, endobronchial component of this patient’s CAO. No risk for airway fire or perforation is present, but cryotherapy can cause cold-induced bronchospasm—a matter of particular risk in our patient with COPD. Cryotherapy has been shown to be most effective when performed in combination with EBRT.27 Similar to PDT and EBB, the effect is delayed and initially might worsen airway obstruction, causing post obstructive pneumonia due to sloughed necrotic tissue.
• Electrocautery and argon plasma coagulation (APC): These techniques remove the exophytic endoluminal component and provide superficial cauterization (3 to 6 mm), which may not suffice to stop large airway bleeding. APC has a risk for gas embolization, which is relevant in our patient because of the vascular pattern of his airway tumor. Argon gas is heavy, inert, and much less soluble in the body than carbon dioxide. As gas is forced into the airway wall, causing perforation, it collects in a blood vessel and passes into the systemic circulation, causing embolism.28 Erosion from tumor also presents a risk to major vessels. Systemic, life-threatening gas embolism has been reported as a complication of endobronchial APC.29
• Fully or partially covered metal stent insertion: This alternative may be used in cases of malignant CAO. In patients with significant comorbidities precluding general anesthesia, it is a suitable alternative in that stents can be placed via flexible bronchoscopy with or without fluoroscopy.30 Metal stents are more costly than silicone stents and can be difficult to remove. Furthermore, a straight stent would not palliate this patient’s lobar bronchial obstruction.
• Flexible bronchoscopy using laser: This procedure could be performed but would be very time-consuming in this patient because of his extensive intraluminal disease. Furthermore, this would only assist with management of the endobronchial component, and stent insertion would still be necessary to palliate extrinsic obstruction. Because of severe bleeding or bronchial obstruction from tissue debris, pulmonary hygiene may be difficult in this patient with COPD.
• Systemic therapies such as EBRT and systemic chemotherapy: These treatments might be appropriate once a final tissue diagnosis is available. If NSCLC is confirmed, this tumor would be a clinical stage IIIB.6 Current evidence shows that for individuals with unresectable disease, a good performance score, and minimal weight loss, treatment with combined chemoradiotherapy results in better survival than radiotherapy alone. Concurrent chemoradiotherapy seems to be associated with improved survival compared with sequential chemoradiotherapy.31 Restoration of airway patency could improve the performance score and accelerate appropriate initiation of systemic therapy. Initiation of EBRT as a primary treatment without attempts to restore airway patency is of doubtful benefit in this patient. EBRT is only variably effective for cancer-induced CAO, especially when CAO results in atelectasis. In a study of 330 patients, EBRT palliated hemoptysis in 84% of patients and superior vena cava syndrome in 86% of patients, but atelectasis in only 23%.32 EBRT after effective laser treatment, however, could potentially improve survival.33 A factor that limits most EBRT treatments is unwanted exposure of the normal lung parenchyma, heart, spine, and esophagus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree