Chapter 1 Rigid Bronchoscopy with Laser Resection for Tracheal Obstruction from Recurrent Respiratory Papillomatosis
Case Description
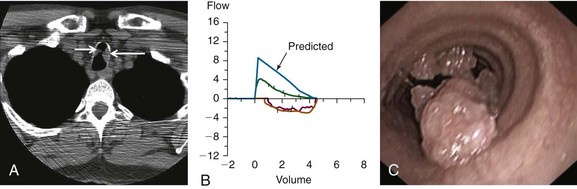
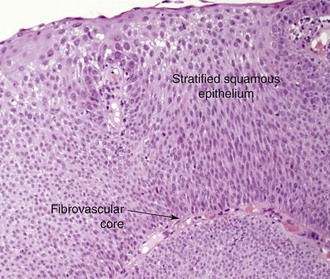
A 53-year-old male patient presented with progressive dyspnea on exertion for 6 months. He had a chronic cough with yellow phlegm but no hemoptysis. The patient was infected with human immunodeficiency virus (HIV) 25 years ago and had been on highly active antiretroviral therapy (HAART), which he was tolerating well. His most recent viral load before presentation was undetectable, and CD4 count was 1200/mm3. He had undergone several laryngeal procedures for laryngeal papillomas 13 years earlier, which resulted in residual hoarseness. His past medical history was significant for chronic obstructive pulmonary disease (COPD), for which he was on albuterol and tiotropium. Neck and chest computed tomography showed two masses in the upper trachea (Figure 1-1, A). He was not married, lived alone, and had a male partner. He worked as a real estate agent and enjoyed his work. He had a 90 pack-year history of smoking but no history of recreational drug or alcohol use. Examination revealed normal vital signs. No wheezing or stridor was observed, but decreased breath sounds were noted bilaterally. Hemoglobin was 15.8 g/dL and white blood cell count was 14,400/mm3. Other biochemical and coagulation markers were normal. Pulmonary function testing revealed a moderate obstructive ventilatory impairment (forced expiratory volume in 1 second [FEV1] of 55% predicted without improvement after bronchodilators), a peak expiratory flow (PEF) of 45% predicted, scooping of the expiratory limb, and flattening of the inspiratory limb on the flow-volume loop (FVL; Figure 1-1, B). Maximal voluntary ventilation was 48% predicted. Residual volume was 130% predicted, and diffusing capacity of the lung for carbon monoxide (DLCO) was 53% predicted. Flexible bronchoscopy revealed two polypoid lesions in the upper trachea (Figure 1-1, C). Biopsy showed squamous papilloma, a central fibrovascular core covered by stratified squamous epithelium, and features of koilocytic atypia and squamous metaplasia but no evidence of malignant transformation. These findings were consistent with his previous diagnosis of recurrent respiratory papillomatosis (RRP) (Figure 1-2).
Discussion Points
1. List four differential diagnoses of exophytic endoluminal tracheal lesions.
2. Describe three indications for adjuvant therapy in recurrent respiratory papillomatosis.
3. Describe the advantages and disadvantages of neodymium-doped yttrium aluminium garnet (Nd : YAG) laser therapy as compared with other laser therapies for treating this patient with RRP.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
The diagnosis of tracheal obstruction was based on nonspecific symptoms and results from chest tomography. Pulmonary function tests showed moderate obstructive ventilatory impairment and mild hyperinflation with reduced DLCO—findings consistent with the patient’s emphysema. The FVL did not reveal a classic pattern of flattening of both inspiratory and expiratory limbs as is seen in patients with fixed central and/or upper airway stenosis; instead, flattening of the inspiratory limb was evident, but the expiratory curve showed only a “scooped out” pattern as is usually seen in asthma and COPD (see Figure 1-1). Interpreting isolated flattening of the inspiratory limb as sign of a variable extrathoracic obstruction1 would be erroneous and inconsistent with this patient’s CT and bronchoscopic findings, which clearly showed intrathoracic obstruction (see Figure 1-1). Flattening of the expiratory limb, as was seen in our patient as well, may be masked by a significant reduction in PEF in patients with COPD.2
In general, the flow-volume loop is an insensitive test for tracheal obstruction because lesions must narrow the tracheal lumen to less than 8 mm before abnormalities can be detected.2 Indeed, reports indicate that exertional dyspnea and reductions in PEF usually occur when the tracheal diameter falls to less than 8 mm.3 In a study of more than 400 FVLs, the sensitivity of several quantitative and visual criteria for upper airway obstruction was 70%.4 Another study showed that in cases of upper airway obstruction (i.e., vocal cord dysfunction), none of the spirometric data predicted disease. Authors concluded that normal FVLs should not influence the decision to perform laryngoscopy.5 Even when the FVL pattern is characteristic, it offers only functional and inexact anatomic (location) information. Thus imaging studies are indicated.
One spirometry test that should not be ignored when patients with suspected tracheal obstruction are evaluated is maximal voluntary ventilation (MVV). The MVV is the largest volume of gas that can be moved into and out of the lungs in 1 minute by voluntary effort with vigorous coaching; normally it is measured as 125 to 170 L/min. The MVV depends on muscular force, compliance of the thoracic wall and lungs, and airway resistance. It is reduced in patients with emphysema or with central airway obstruction. A reduction in MVV, however, is nonspecific and is caused by upper or lower airways obstruction, restriction, or muscle weakness.6 In a patient such as ours who showed good effort during the MVV maneuver and had no evidence of neuromuscular disease or restriction, the suspicion for airway obstruction is high. Although MVV is reduced in emphysema, a disproportionate reduction in measured MVV compared with the estimated value (MVV/FEV1 of less than 25, such as that seen in our case), in fact, has a sensitivity of 66% for diagnosing upper airway obstruction.7
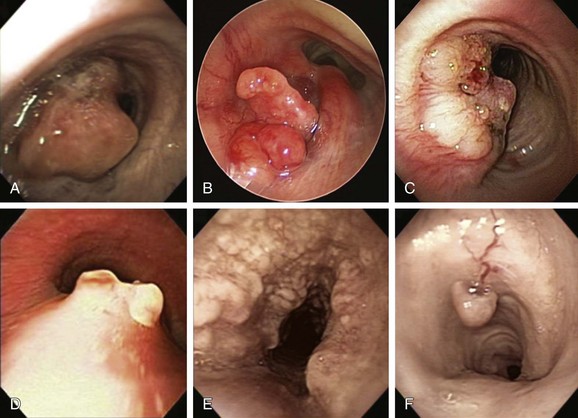
This patient had received a diagnosis of RRP and in the past required several procedures for laryngeal papillomas. In view of this, the likely diagnosis for the tracheal polypoid, “cauliflower-like” lesions was RRP. The differential diagnosis of this exophytic endoluminal lesion includes malignant and other benign processes. Tracheal malignant tumors are rare, constituting only 2% of all respiratory tract tumors.8 These most commonly include squamous cell carcinoma and adenoid cystic carcinoma, which are responsible for 70% to 80% of tracheal tumors. Other tracheal tumors include carcinoid tumors, mucoepidermoid carcinomas, and a wide variety of carcinomas, sarcomas, lymphomas, and plasmacytomas.9 Among lesions of sufficient severity to require intervention, malignant lesions have accounted for between 25% and 66% of cases; one third have been primary lesions and two thirds were secondary.10 Cancers that can directly invade or metastasize to the airway and cause tracheal obstruction include renal cell, esophageal, lymphoma, melanoma, breast, colon, and thyroid carcinomas (Figure 1-3). For these reasons, biopsy is warranted to confirm diagnosis, even when “classic” polypoid, “cauliflower”-like lesions are seen during bronchoscopy.
Benign tumors account for less than 10% of tumors involving the trachea and mainstem bronchi.11 Among histologically benign causes of tracheal exophytic endoluminal lesions, one should consider granulation tissue from endotracheal or tracheostomy tubes, airway stents, foreign bodies, hamartomas, solitary papillomas, lipomas, leiomyomas, chondromas, amyloidosis, exuberant tracheopathica osteochondroplastica, and inflammatory myofibroblastic tumor12 (see Figure 1-3). Overall, most respiratory tract tumors are malignant, and benign tumors are rare (approximately 1.9% of all lung tumors); most of these are papillomas and hamartomas.13
Although RRP is considered by some investigators to be an uncommon tumor, secondary to infection with human papillomavirus (HPV) types 6 and 11, it is actually the most common benign tracheal neoplasm.14 In our patient, the tracheal papillomas probably represented spread of disease from the original laryngeal site. The rate of tracheal involvement by laryngeal papilloma has been reported in the literature to be 2% to 17%.15 Once in the tracheobronchial tree, RRP is difficult to control, causes significant morbidity, and in almost 2% of cases may undergo malignant transformation.16 Malignant degeneration is aggressive and often is rapidly fatal, but it occurs infrequently in the absence of prior radiation therapy.17
Comorbidities
The patient’s comorbidities included moderate COPD and HIV infection. If interventions were provided with the patient under general anesthesia, these comorbidities could significantly increase the risk for COPD exacerbation or postoperative pneumonia. In a large retrospective study, however, HIV-infected patients were matched 1 : 1 with HIV-seronegative patients undergoing surgical procedures by type, location, age, and gender; findings showed that clinical outcomes, length of stay, and number of postoperative visits were similar among the matched patient pairs. Various complications were no more frequent among HIV-infected patients, except for pneumonia. Among the HIV-infected group of patients, a viral load of 30,000 copies/mL or greater was associated with a threefold increased risk of complications, but a CD4 cell count <200/mm3 was not associated with increased risk.18 Our patient’s CD4 count was greater than 1000 and the viral load was undetectable, putting him in a low-risk group for developing postoperative pneumonia.
A patient’s COPD should be treated so the best possible baseline level of function can be achieved before elective interventions are provided. A retrospective study of patients with COPD undergoing general anesthesia illustrated the importance of optimizing preoperative function.19 In this report, 227 of 464 patients underwent some sort of preoperative preparation, including various combinations of bronchodilators, antibiotics, and systemic glucocorticoids. The incidence of pulmonary complications was lower in the prepared group than among those receiving no preoperative preparation (23% vs. 35%). Another study noted a reduction in the incidence of pulmonary complications from 60% to 22% in a group of high-risk patients prepared with bronchodilators, smoking cessation, antibiotics, and chest physical therapy.20 Our patient had stable moderate COPD at the time of evaluation and was treated with short-acting β2-agonists and long-acting anticholinergic agents according to international guidelines.21
Support System
This patient was living with HIV infection. Several of the attributes of HIV illness increase the likelihood that its victims will be stigmatized, for example, the illness is viewed in society as the result of individuals violating the moral order; the contagiousness of HIV is perceived to threaten society; HIV illness is viewed as a debilitating disease that results in death; and this disease has most frequently been associated with groups already marginalized in society. Of course, the HIV-acquired immunodeficiency syndrome (AIDS) stigma has the potential to influence health and health-seeking behaviors in a variety of ways and, therefore, should be an important consideration for health care professionals. Studies show disempowering health care practices occur within the health care encounter when persons living with HIV access health services.22 The dominant and powerful role of health care professionals (in particular physicians) in the treatment decisions of persons living with HIV has been documented. Medical surveillance of an individual after an HIV-positive diagnosis was considered by some a “manifestation of paternalistic power in the guise of knowledge-seeking and in the name of beneficence.”23
Our patient had a male partner who seemed very supportive. Study findings show heterogeneity in dyadic (i.e., relational level) support for illness management. In the context of HIV, a patient’s social support may be particularly important in terms of adherence to medications.24 Strict HAART adherence is required for treatment success and increased survival in patients living with HIV. Nonadherence can increase the risk of developing drug-resistant viral strains and transmitting drug-resistant strains to others. Regarding RRP, nonadherence could result in an inability to control the disease when adjuvant therapies are necessary. Although family and friends frequently provide support, relationship partners are a primary source of social support for gay male couples coping with HIV.25
Patient Preferences and Expectations
This patient had no evidence of cognitive dysfunction and was able to clearly express his desire for treatment. His partner was involved in these conversations per the patient’s request, and they agreed to proceed with available therapeutic options for tracheal papillomatosis.* Thus rigid bronchoscopy under general anesthesia was offered to this patient.
Procedural Strategies
Indications
Although no treatment has been consistently shown to eradicate RRP, removal of papilloma tissue as completely as possible without compromising normal airway wall structures may reduce recurrence and risk for malignant transformation. The pattern of obstruction was exophytic intraluminal, and no evidence of extrinsic compression was found. For endoluminal central airway obstruction, bronchoscopic therapies include electrosurgery, laser resection, microdebridement, rigid bronchoscopic debulking, cryotherapy, brachytherapy, and photodynamic therapy. No stent insertion was planned unless airway lumen narrowing remained at 50% or greater.26 Adjuvant treatments include potentially curative gene therapy (epidermal growth factor receptor [EGFR] tyrosine kinase inhibitors), retinoids (oral metabolites or analogs of vitamin A), and intralesional injection of antiviral agents in an attempt to induce growth arrest or apoptosis, or to inhibit the proliferation or promote the normal differentiation of HPV-infected cells.27
Contraindications
No absolute contraindications to rigid bronchoscopy were noted. However, the risk of perioperative cardiac complications should be considered in this patient with a history of HIV infection because diabetes mellitus, dyslipidemia, and coronary atherosclerosis are increasingly common among HIV-infected patients on long-term antiretroviral therapy.28 One study found electrocardiographic (ECG) evidence of asymptomatic ischemic heart disease in 11% of HIV-infected patients.29 Our patient had no clinical or electrocardiographic signs of coronary artery disease and had been cleared for general anesthesia by his internist.
Expected Results
Rigid intubation was planned using a 12-mm-diameter Efer-Dumon nonventilating rigid bronchoscope (Efer, La Ciotat, France) to allow passage of laser fiber, a rigid suction catheter, and forceps. Nd : YAG laser photocoagulation followed by rigid bronchoscopic debulking under general anesthesia was planned, along with spontaneous assisted ventilation. The goal was to reduce tumor burden, restore airway patency, and improve dyspnea, thus eventually decreasing regional dissemination of disease.16
Removal of HPV-involved tissues as completely as possible and without compromise of normal airway structures appears necessary to reduce recurrence. Most studies performed by otolaryngologists evaluated carbon dioxide (CO2) or potassium-titanyl-phosphate (KTP) lasers because the disease is more commonly localized in the larynx. However, many reports have described successful use of Nd : YAG laser resection for RRP, especially when the trachea is involved.30–34 One case series, for example, evaluated five patients with RRP; none had recurrence of disease after 1 year of follow-up post Nd : YAG laser treatment.30 In urology, for instance, the Nd : YAG laser was used to effectively treat HPV-associated genital papillomas (caused by HPV 6 and HPV 11); its use led to a lower rate of recurrence compared with CO2 laser treatment after 1 year of follow-up.35 Moreover, tissue biopsies after Nd : YAG laser surgery demonstrated HPV recurrence mainly in nontreated areas, whereas after CO2 laser treatment, viral recurrence was observed within and at the margins of treated tissue. This might be attributed to the fact that, in comparison with vaporizing (what you see is what you get) CO2 laser energy, Nd : YAG laser energy provides deeper (what you don’t see might hurt you) coagulation along with destruction of the HPV-infected basal cell layer of the mucosa. This region is usually responsible for the regeneration of papilloma tissue.35 Nd : YAG laser coagulation of papilloma tissue in a noncontact mode may cause less smoke-containing toxic pyrolysis products and infectious HPV particles, and could potentially lower the risk of HPV transmission to adjoining healthy tissue compared with CO2 laser surgery. In addition, effective suctioning during rigid bronchoscopy with the Nd : YAG laser offers fast and efficient removal of the unavoidable but small amount of potentially infectious laser plume. This might be another reason for the low rate of recurrence in a study of RRP lesions treated with the Nd : YAG laser.30
Team Experience
Nd : YAG laser treatment of RRP should be provided by physicians who are experienced in the application of noncontact Nd : YAG laser and able to estimate the thermal impact on treated tissue. The operator who is not aware of injury to deeper tissue layers caused by injudicious laser usage may encounter unacceptable scarring or even airway perforation and massive bleeding. Inappropriate and aggressive use of the laser may cause injury to nonaffected adjacent tissues and may create an environment suitable for implantation of viral particles. Procedures should not be performed in a facility that does not have the necessary complement of equipment for safe instrumentation of a patient’s airway.36
Risk-Benefit Analysis
Although Nd : YAG laser may cause deep tissue damage, our patient had symptoms that required restoration of airway patency. No risk-benefit analysis has been performed to compare Nd : YAG laser versus other types of lasers or other treatment modalities, but several alternative techniques have been proposed for treating RRP. One survey showed that the microdebrider and the CO2 laser were the preferred means for removal of laryngeal RRP; 52.7% of respondents preferred the microdebrider, and 41.9% the CO2 laser.37
Therapeutic Alternatives for Restoring Airway Patency
• CO2 laser vaporization: done under general anesthesia usually with muscle relaxants, with high-frequency supraglottic jet ventilation, and under suspension micro-laryngotracheoscopy. The CO2 laser is believed to enhance precision and is preferred by some surgeons because of its short extinction coefficients and minimal thermal injury to adjacent tissues. The CO2 laser has an emission wavelength of 10,600 nm and converts light to thermal energy that is absorbed by intracellular water; the result is controlled destruction of tissues by cell vaporization and cautery of tissue surfaces with minimal bleeding. Its use through a flexible bronchoscope has been described, but usually the CO2 laser has to be coupled to an operating microscope, which allows treatment only with a rigid system; a high level of expertise and good coordination are needed to reach all affected areas while avoiding injury to healthy tissue adjacent to the papillomas. In one series of 244 patients with RRP treated over 2 months with the CO2 laser, “remission” was achieved in 37%, “clearance” in 6%, and “cure” in 17% of cases.38 However, CO2 laser surgery may result in dissemination of infectious viral particles included in the laser plume with the potential for harmful effects on operating room personnel and patients.39
• Microdebrider: used by otolaryngologists as a laryngeal shaver for RRP. Advocates of this technique claim that the shaver is safer and more accurate and prevents thermal injury, and that postprocedure edema associated with use of the laser is minimized because tissue injury resulting from the shaver technique is confined to the superficial mucosa.40 Some investigators used an endoscopic microdebrider to quickly debulk laryngeal disease. Pasquale et al. reported improved voice quality, less operating room time, less mucosal injury, and a cost benefit when the microdebrider was used compared with the CO2 laser.41 A Web-based survey of members of the American Society of Pediatric Otolaryngology found that most respondents favor the use of “shaver” technology.37 Safety advantages include no risk of laser fire or burns and apparently no risk of aerosolized viral DNA particles. However, debilitating injury and scar with subsequent dysphonia have been reported.42
• The KTP laser with a 532 nm wavelength is very useful for cutting and coagulating tissues simultaneously; its incisional strength does not penetrate as deeply as the Nd : YAG laser, so less collateral tissue damage occurs. The KTP laser has been used successfully in treatment for tracheal papillomas.32 Zeitels et al. reported that the use of a 532 nm pulsed KTP laser in the treatment of recurrent glottal papillomatosis and dysplasia led to 75% regression of disease in two thirds of patients; good results were also reported with a solid-state fiber-based thulium laser that functions similarly to a CO2 laser, with the benefit that the laser beam is delivered through a small glass fiber.43
• Pulsed-dye lasers (wavelength 577 and 585 nm) are reportedly feasible and safe for treating patients with RRP44; McMillan et al. reported good preliminary results in three patients with use of the 585 nm pulsed-dye laser.45 Rees et al. performed 328 pulsed-dye laser treatments in the office in 131 adult patients with upper airway RRP and reported that patients overwhelmingly preferred in-office surgery to a procedure received under general anesthesia.46
• Argon plasma coagulation (APC): allows controlled, limited penetration into tissues and good control of bleeding without carbonization or vaporization. APC has been used for RRP with good control of disease and no side effects or complications.47
• Silicone stent insertion may be useful in refractory endobronchial RRP when medical and other endobronchial therapies fail to restore airway patency. Case reports show that papilloma debulking and silicone stents can offer adequate control of symptoms.48
• Tracheostomy sometimes is performed to provide a secure airway for patients who require weekly or monthly surgical procedures (especially for laryngeal disease). It is noteworthy, however, that approximately 50% of tracheotomized patients develop peristomal and distal tracheal papillomas.49
• Adjuvant therapy: The decision to initiate adjuvant therapy should be individualized according to the frequency of surgical interventions, the morbidity of frequent surgeries, and the recurrence pattern of the papillomas. It has been suggested that adjuvant therapies are needed if surgery is required more frequently than 4 times a year for 2 years, or if papillomas begin to spread outside of the endolarynx. Adjuvant therapies include α-interferon, acyclovir, indole-3-carbinol, retinoic acid, photodynamic therapy, ribavirin, cidofovir, and cimetidine. Of note, few of these therapies have been evaluated in randomized prospective trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree