Chapter 21 Rigid Bronchoscopic Tumor Debulking and Silicone Stent Insertion for Mixed Malignant Tracheal Obstruction Caused by Esophageal Carcinoma
Case Description
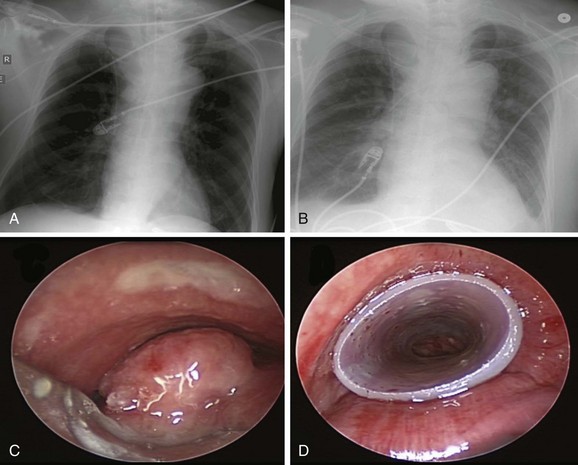
Chest radiograph showed a soft tissue mass density projecting to the right of the trachea. Lung volumes appeared to be enlarged bilaterally (Figure 21-1). Laboratory markers revealed WBC 21.3, Hb 12, and Plt 164. Results from the esophageal mass biopsy showed squamous cell carcinoma. Ventilator settings included assist-control volume-control ventilation with a tidal volume of 500 mL, rate 16, PEEP 5, and FiO2 0.5. At these settings, peak airway pressure was 60 cm H2O and plateau pressure 28 cm H2O. Weaning was attempted but failed. Rigid bronchoscopy was performed, revealing extrinsic compression and an exophytic endoluminal mass protruding from the posterior wall, completely occluding the mid-trachea (see Figure 21-1). The mass was 2 cm in length and was located 5 cm above the carina and 5 cm below the vocal cords. The endoluminal tumor was cored out using Nd:YAG laser for hemostasis. Airway patency was improved, but tracheal obstruction from extrinsic compression (>70%) remained significant. A 16 × 50 mm straight studded silicone stent was therefore placed in the mid-trachea, restoring airway patency satisfactorily. The patient was successfully extubated the next day (see Figure 21-1).
Discussion Points
1. Describe three patient-related factors associated with difficult rigid bronchoscopic intubation.
2. Explain the rationale for stent insertion after tumor debulking in this patient.
3. List at least three strategies to prevent airway perforation and hemorrhage during rigid bronchoscopy.
4. Describe five ways to avoid complications of rigid bronchoscopy.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
Advanced, unresectable esophageal cancer with airway invasion has a very poor prognosis (5 year survival rate <10%).1 Patients with this disease have not only a limited life expectancy but also many potentially debilitating complications from the local effects of their tumors. These include dysphagia, dysphonia, dyspnea from airway involvement, chest pain, and, rarely, massive fatal hemorrhage from aortic erosion.2,3 Airway involvement in the setting of esophageal carcinoma includes tissue invasion, infiltration, or the presence of an esophago-respiratory fistula (ERF).* Airway complications may be caused (1) by extrinsic compression on the airways by the tumor or by an esophageal stent and (2) by direct invasion by the tumor into the airways. These processes can result in severe airway narrowing or ERF. Among a large series of 372 patients seen over the 14 year study period, 74 (20%) were found to have airway involvement: 35 (47%) of these patients had an ERF; in the absence of ERF, airway involvement identified as invasion or infiltration was biopsy proven in 29 of 39 patients (74%).4 Other investigators report airway involvement in 10.9% to 62% of cases.4,5 Because of the proximity of the major airways to the upper two thirds of the esophagus in the thorax, major airway obstruction is also common. In fact, patients with esophageal tumors above the level of the main carina have a worse prognosis than those with lower tumors because of this anatomic relationship with the large airways.6 Hence, up to 25% of patients with advanced esophageal cancer may require airway stent insertion to alleviate airway obstruction.7–10 The mean duration of survival for patients requiring airway stent insertion for obstruction due to esophageal cancer reportedly ranges from only 35 to 121 days, with many patients dying at home or in hospice.9
Severe respiratory symptoms in these patients may occur because of ERF or severe airway narrowing. For tumors extending into the airway lumen, as is seen in our patient, primary goals of therapy include palliative relief of the malignant obstruction of the esophageal lumen and central airway. If present, attempts can be made to close fistulas between the esophagus and the central airway or, at the least, to alleviate or diminish risks of aspiration and inability to swallow. Recurrent aspiration pneumonia may be caused by dysphagia due to esophageal obstruction or by ERF and can lead to respiratory failure. Initial chest radiographs are often normal; in these cases, they are useful for excluding other causes of respiratory symptoms such as pneumonia, pneumothorax, and pleural effusion.11 In our patient, for example, pneumonia was not evident on chest x-ray (CXR) nor was ERF on bronchoscopy. The critical airway obstruction was therefore the likely culprit for respiratory failure in view of notably high peak airway pressures and normal plateau pressures, which point to an airway resistance process. In the absence of evidence for bronchospasm, mucus plugging, or kinking of the endotracheal tube, the most plausible explanation for high peak airway pressures and respiratory failure in this patient was tracheal obstruction by the esophageal mass. This was probably worsened by the moderate sedation used for EGD.
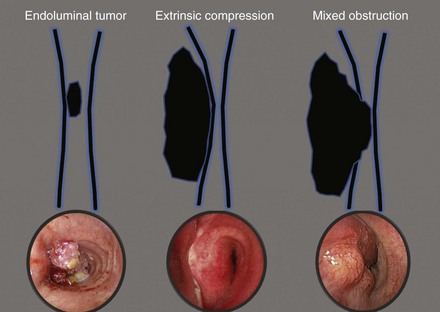
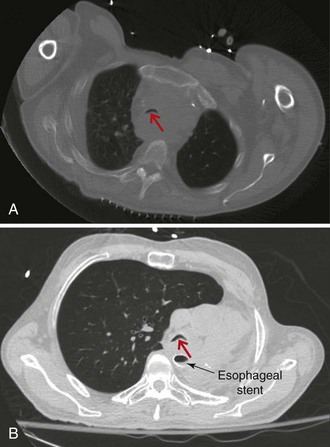
On bronchoscopy, this patient’s central airway obstruction was classified as mixed because it comprised an endoluminal component and extrinsic compression (Figure 21-2).12 In addition to bronchoscopy, computed tomography (CT) provides a noninvasive means of confirming the presence and severity of suspected airway involvement and is valuable in assessing the length of a stenosis, especially when a bronchoscope may not be passable beyond the stenosis. Before bronchoscopy, CT may help to determine whether double stent insertion is necessary (Figure 21-3). In cases of suspected ERF, a barium swallow is usually performed.
Comorbidities
It is possible that our patient had hypoventilation post EGD, caused by sedation-induced reduction in minute ventilation in the setting of critical tracheal obstruction, or possibly by upper airway obstruction unmasked by sedatives. The redundant pharyngeal and laryngeal tissues, as seen in patients with obstructive sleep apnea (OSA), may further lose tonicity, contributing to the hypoventilation and hypoxemia induced by moderate sedation, which potentially leads to respiratory failure and endotracheal intubation (see video on ExpertConsult.com) (Video V.21.1![]() ). Although not documented in his medical history, based on the bronchoscopic findings, we suspected OSA in our patient. This can also contribute to poor outcomes caused by perioperative complications. Indeed, evidence suggests that patients with OSA may have more postoperative hypoxemia, upper airway obstruction, cardiac arrhythmias, and myocardial infarction.13 Results of observational studies show that most patients with OSA who are undergoing surgery have not been diagnosed before surgery. In a study of 2877 elective surgery patients, 24% were found to be at risk for having OSA, and 81% of these had not been diagnosed previously.14 These data raise the question whether all patients undergoing surgery should be screened for OSA.
). Although not documented in his medical history, based on the bronchoscopic findings, we suspected OSA in our patient. This can also contribute to poor outcomes caused by perioperative complications. Indeed, evidence suggests that patients with OSA may have more postoperative hypoxemia, upper airway obstruction, cardiac arrhythmias, and myocardial infarction.13 Results of observational studies show that most patients with OSA who are undergoing surgery have not been diagnosed before surgery. In a study of 2877 elective surgery patients, 24% were found to be at risk for having OSA, and 81% of these had not been diagnosed previously.14 These data raise the question whether all patients undergoing surgery should be screened for OSA.
Patient Preferences and Expectations
The prognosis was discussed with the patient’s wife. By investing in patient-centered conversations with family members, health care providers can better meet the needs of families and patients during a medical crisis such as respiratory failure or admission to the intensive care unit.15 His wife hoped he could be weaned from the ventilator, so she could speak with him about his wishes.
Procedural Strategies
Indications
The primary goal was to restore airway patency to facilitate extubation and change the level of care. Interventional bronchoscopic procedures have been reported to facilitate weaning from mechanical ventilation.16 Relevant to this patient’s condition, in a study of 11 inoperable patients with esophageal carcinoma and airway involvement treated with a combination of rigid bronchoscopy, neodymium-doped yttrium aluminum garnet (Nd:YAG) laser resection, and silicone stent placement, authors noted that 4 patients who required mechanical ventilation for respiratory failure were successfully weaned from mechanical ventilation after the procedure.9 Overall, airway stent insertion is considered a palliative intervention for airway complications in patients with unresectable esophageal cancer.9 Airway stents are effective for treating airway narrowing from both extrinsic compression and direct tumor invasion and have been shown to be useful in the treatment of ERF, if present.4,17,18 The choice of stent depends on the clinician, the place of practice, financial or health care social policy circumstances, institutional biases, and equipment availability, as well as the particular needs of the individual patient. Clinician factors include personal training, familiarity with rigid and flexible bronchoscopy, and personal preference. Institution-related factors include costs (self-expanding metallic stents [SEMS] are generally more expensive), availability of operating theaters and anesthesiologists for emergency rigid bronchoscopy, and stent availability. In terms of patient factors, one might consider the fitness of the patient for general anesthesia, the risk of provoking complete airway occlusion outside of an operating theater while performing flexible bronchoscopy, and any foreseeable chance that a stent may need to be adjusted or replaced subsequently.11
Expected Results
Stent insertion in the involved airway of esophageal cancer has been shown to improve the life quality and outcomes of patients with advanced esophageal cancer.4 In the largest case series to date, 66 airway stents (65 studded silicone stents and 1 Wallstent metal stent) were inserted in 51 patients with airway involvement from esophageal carcinoma. Forty stents were inserted in the trachea, 16 in the left main bronchus, and 10 in the right main bronchus. In 47 patients (92%), improvement in respiratory symptoms was significant. The mean survival was 107.7 days.8
Although some might say that bronchoscopic stent insertion is an unwarranted and potentially costly therapeutic alternative with patients with ultimately and rapidly fatal disease, we contend that stent insertion is a palliative intervention that always warrants consideration, not only because it results in greater comfort, even over the short term, but also because it improves quality of life, might allow withdrawal from mechanical ventilation so that patients can communicate more fully with their loved ones, and may result in a reduction in level of care or more rapid discharge from the intensive care or hospital setting. In the Netherlands, where euthanasia is a legal alternative to certain palliative therapies for patients with advanced cancer, 7 out of 12 patients with esophageal cancer who elected to have airway stents inserted were judged by their family physicians to have received “worthwhile” palliation in terms of quality of life during the terminal phase of their disease, despite their relatively short remaining survival time.19
Team Experience
In high esophageal tumors and in locally invasive tumors with evidence of ERF, a shared interdisciplinary care approach between gastroenterology and pulmonary teams is recommended.20 The type of airway stent selected may depend on the level of team experience and training in the technique of rigid bronchoscopy.21 Bronchoscopic resectional techniques such as laser, electrocautery, cryotherapy, argon plasma coagulation (APC), and photodynamic therapy (PDT), in addition to metal or silicone stent insertion, were available at our institution. Therefore in our case, the choice of treatment was guided by (1) a need for immediate improvement in airway patency to facilitate extubation, (2) a need to maintain airway patency in view of extrinsic compression, and (3) a desire for a stent that could be removed or changed easily in case of changes in clinical illness. Thus we elected to perform our procedure using a resection technique such as Nd:YAG laser for possible hemostasis and photocoagulation of tumor in conjunction with coring out, and to insert a silicone rather than a covered metal stent because of its greater expansile force.22,23
Therapeutic Alternatives for Restoring Airway Patency
Alternative therapeutic modalities include insertion of SEMS, radiation therapy, chemotherapy, surgical intervention (esophageal bypass, diversion, and attempted resection), and comfort measures. This patient was not considered a candidate for surgical intervention given his advanced disease, poor functional status, and respiratory failure. Less invasive palliative interventions considered included other palliative bronchoscopic options such as mechanical debridement, dilation, laser ablation, electrocautery, cryotherapy, photodynamic therapy, and brachytherapy alone.24 Without stent insertion, however, restoration of airway patency may not be immediate or long-lasting. In addition, any remaining extrinsic compression after debulking would require stent placement to maintain patency.
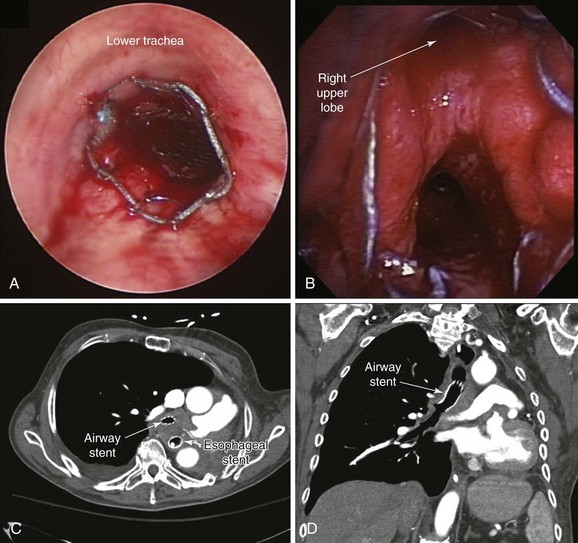
1. Covered self-expandable metallic stents (SEMS) have been used to relieve the airway obstruction and seal ERFs to avoid aspiration symptoms.10,17 Insertion of SEMS in mechanically ventilated patients could be performed via rigid bronchoscopy under general anesthesia (Figure 21-4) or via flexible bronchoscopy under fluoroscopic guidance.25 Some patients are not suitable for rigid bronchoscopy with a general anesthetic because of the severity of their illness, comorbidities, or refusal to undergo intervention. Fluoroscopy requires special facilities that may not be available in every intensive care unit (ICU). In the absence of ready availability of an operating room, and for those operators not versed in the techniques of rigid bronchoscopy,21 this procedure can, however, be performed while the patient is on the ventilator in the ICU. Because a significant part of the obstruction was caused by malignant extrinsic compression in our patient, stent insertion could have restored airway patency with or without minimal flexible bronchoscopic debulking of the mass using electrocautery or APC. A technique for placing these stents without fluoroscopy includes the following steps: The bronchoscope is inserted into the mouth through a bite block alongside the endotracheal tube (ETT) and is advanced into the space between the tracheal wall and the endotracheal tube; the scope is then positioned proximal to the lesion; a guidewire is inserted through the bronchoscope and is passed alongside the lesion, after which the bronchoscope is withdrawn, leaving the guidewire in place. The scope is reinserted into the ETT to confirm guidewire location. A stent delivery catheter is advanced over the guidewire, and the stent is deployed under bronchoscopic visualization. The delivery catheter and the guidewire are withdrawn together, with the stent left in position (Figure 21-5). If necessary, the stent can be repositioned by grasping its proximal loop with a flexible alligator forceps (see video on ExpertConsult.com) (Video V.21.2![]() ).
).
2. Definitive chemo-radiotherapy has been proposed for patients with esophageal cancer and airway invasion with or without ERF. Radiation therapy is effective in palliating dysphagia in 34% to 48% of patients with inoperable esophageal cancer.26 Concurrent chemotherapy with radiotherapy affords even greater benefit in terms of survival and loco-regional control,1 although it is associated with significant treatment-related morbidity and mortality. Anecdotal evidence suggests a clinical complete response with induction chemotherapy followed by consolidation with concurrent chemo-radiotherapy—an approach that may reduce the morbidity of upfront radiation.27 Progression to ERF, however, has been reported with radiotherapy; in one case series, the incidence was approximately 10% (9 of 85 patients).28 This palliative intervention was not considered a first step in our patient given his poor performance status and the presence of respiratory failure.
3. Double stent insertion can effectively palliate both dyspnea and dysphagia. Several reports have described the use of “double” stenting of the esophagus and the airway. Results in terms of immediate relief of respiratory and swallowing symptoms appear to be excellent. Esophageal stents, however, are known to compress the adjacent airway, precipitating or exacerbating airway narrowing (see Figure 21-3).7,29 Acute airway obstruction accompanied by stridor, respiratory failure, and death has been reported after placement of esophageal stents in the proximal one third of the esophagus.7,30 Double stenting of the esophagus and the airway has been described to manage this complication,7,8,17 particularly when malignancy obstructs the lumina of the esophagus and the tracheobronchial tree. The incidence of airway obstruction following esophageal stent insertion ranges from about 1% to 10%.9 Airway stent insertion effectively restores airway patency in this setting.7 Airway stent insertion should always be considered when the esophagus requires stent placement, and certainly respiratory compromise accompanying or following esophageal stent insertion should raise the possibility of airway obstruction by the stent itself. A multidisciplinary approach including an initial airway evaluation for patients being considered for esophageal stent insertion improves prognosis and decreases airway complications related to the esophageal stent.31 The double stenting procedure is not without complications, however. When double stenting is used in combined esophago-airway lesions, massive bleeding is reported in up to 27%.32 Double stenting is associated with ERF in up to 18% to 38% of patients with esophageal cancer,29,32 although it is unclear whether fistulas occur as a direct result of stent-related erosion of altered neoplastic infiltration or airway and esophageal mucosa, or if they are a natural result of disease progression.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree