Chapter 28 Rigid Bronchoscopic Intervention for Central Airway Obstruction and Concurrent Superior Vena Cava Syndrome Caused by Small Cell Carcinoma
Case Description
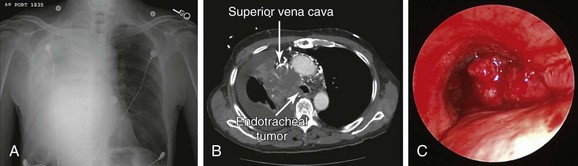
An 85-year-old male with an extensive history of smoking (70 pack-years) developed shortness of breath, which worsened within the week before admission. He had excessive cough, which resulted in hemoptysis (estimated at a few teaspoons/day). Review of systems revealed weight loss (20 kg/6 mo), as well as facial and neck edema for several months. Vital signs showed blood pressure of 150/70 mm Hg, heart rate of 115/min, body temperature of 37.2° C, and respiratory rate of 22/min. On physical examination, the patient had prominent edema of the face, neck, and bilateral upper extremities, with neck vein distention and multiple engorged dilated vessels over the anterior aspect of the chest. Expiratory wheezing was heard on the left hemithorax, and no breath sounds were heard on the right. The rest of the physical examination was normal. Laboratory findings showed WBC count of 19,700 (neutrophils 81.3%, lymphocytes 2%), hemoglobin of 12.8 g/dL, and platelet count of 310,000/mm3. Arterial blood gas analysis showed pH of 7.54, arterial carbon dioxide tension (PaCO2) of 39 mm Hg, and partial pressure of oxygen in arterial blood (PaO2) of 64 mm Hg (O2 = 2 L/min on nasal prong). Electrolytes were within normal limits. Electrocardiography (ECG) showed sinus tachycardia with bifascicular block. Two-dimensional echocardiography showed a small secundum-type atrial septal defect, normal left ventricular function, and no evidence of right ventricular dysfunction. Chest radiograph revealed near complete opacification of the right hemithorax (Figure 28-1). Chest computed tomography showed a 7.3 × 5.7 cm large mediastinal and right hilar mass with near complete occlusion of the superior vena cava (SVC); the mass had eroded into the right mainstem bronchus and lower trachea, causing near complete collapse of the right lung and a right pleural effusion (see Figure 28-1). Bronchoscopic biopsy and washings performed at an outside facility showed small cell carcinoma. The patient was placed on broad-spectrum antibiotics and was transferred to our hospital for consideration for bronchoscopic intervention to restore airway patency. On repeat bronchoscopic examination, the tumor involved the lower trachea above the main carina, completely occluding the entrance to the right main bronchus (see Figure 28-1). The patient was a retired clothing factory owner who lived with his wife. He had a very close family including several children who were actively involved in his care. His family wanted him to receive active and what was hoped would be effective treatment for this tumor. Emergency radiotherapy of first intention had not been recommended by a radiation oncologist because of concerns for worsening tracheal obstruction by radiation-induced edema and ongoing sepsis. Therefore urgent rigid bronchoscopy was scheduled to establish airway patency and to potentially avoid worsening sepsis and respiratory failure.
Discussion Points
1. List two major complications during general anesthesia in patients with large mediastinal masses.
2. Comment on the safety of therapeutic rigid bronchoscopy in people 80 years of age and older.
3. Enumerate seven measures to reduce operative and anesthetic complications in patients with concurrent superior vena cava syndrome and central airway obstruction.
4. Describe three major complications during rigid bronchoscopy in patients with large carinal tumors completely occluding a mainstem bronchus.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
This patient had a new diagnosis of small cell lung cancer (SCLC). The Veterans Affairs Lung Study Group (VALSG)* staging system has been used traditionally to stage SCLC because of its simplicity. Based on this system, limited disease is seen in 30% to 40% at presentation, and extensive disease in 60% to 70% of patients. Accurate staging is clinically relevant because patients with limited-stage disease are treated with combined modality therapy, and those with extensive disease receive chemotherapy alone. Staging by the VALSG system is controversial in patients with locally advanced disease such as contralateral hilar or supraclavicular nodes, pericardial effusions, or malignant pleural effusions, as were seen in this case; for instance, this group is neither precisely defined (as limited or extended disease) nor uniformly managed by different investigators and is frequently excluded from protocols for limited-stage disease. In this regard, the consensus report from the International Association for the Study of Lung Cancer (IASLC) modified the VALSG classification based on the tumor-node-metastasis (TNM) staging system, and only patients with TxNxM1 were considered as having extended disease, so that IASLC criteria include more patients in the prognostically superior limited disease category than are assigned by the VALSG criteria.1 The TNM staging system used for non–small cell lung cancer (NSCLC) has been increasingly advocated by the IASLC to stage SCLC because it describes the extent of disease more accurately than the VALSG system. In fact, the new IASLC M1a descriptors (pleural effusion, pericardial effusion, and contralateral/bilateral intrapulmonary metastasis) adequately prognosticate SCLC patients as having metastatic disease. In fact, the IASLC recommends the use of TNM for all cases of SCLC.2
Similar to our case, patients with advanced-stage lung cancer of any type may present with a variety of loco-regional complications, including central airway obstruction (CAO), superior vena cava (SVC) syndrome, hemoptysis, and post obstructive pneumonia. Patients with CAO usually are not candidates for surgical resection for physiologic or oncologic reasons. Furthermore, chemotherapy and/or radiotherapy in the setting of post obstructive pneumonia may exacerbate the risk for sepsis. The prognosis is guarded, and in the presence of atelectasis, the ability of external beam radiation alone to restore airway patency was shown to be as low as 23%.3 SVC obstruction by lymph node metastasis into the right paratracheal or precarinal station or by direct invasion of lung cancer can cause SVC syndrome* in up to 10% of newly diagnosed cases of SCLC.4 Tumor growth in most cases is gradual, allowing sufficient time for collateral circulation to develop, but many patients eventually present with headache, swelling of the face and neck, and even coma. However, SVC syndrome is no longer considered an emergency, and the use of intravascular stents is recommended only for relapsed or persistent SVC obstruction following chemotherapy or radiation therapy in SCLC.5
Comorbidities
SCLC is the most common malignancy associated with neurologic paraneoplastic syndromes produced by autoantibodies that cross-react with both SCLC cells and the central nervous system or the neuromuscular junction. These antibodies can cause the Lambert-Eaton myasthenic syndrome (LEMS)† in 3% of patients suffering from SCLC.6 Our patient had no obvious neurologic symptoms suggesting LEMS or other paraneoplastic neurologic syndromes seen in SCLC such as limbic encephalitis,‡ paraneoplastic cerebellar degeneration, autonomic neuropathy, or subacute peripheral sensory neuropathy. SCLC cells can also produce a number of polypeptide hormones, including adrenocorticotropic hormone (ACTH) and antidiuretic hormone, resulting in the syndrome of inappropriate antidiuretic hormone and Cushing’s syndrome, respectively. Our patient had no symptoms or laboratory markers suggesting these diagnoses, all of which could affect anesthesia and procedural planning.
Support System
Cancer treatment is emotionally and physically exhausting for patients, so it is important for them to have a good support system during this critical time of their lives. This patient had many family members who were eager to help. Strong family support and faith are noted to have a positive effect on response to cancer treatment.7 If patients, friends, or family members have difficulty coping with the emotional aspects of the illness, experienced professionals in mental health services, social work services, and pastoral services, and local support groups can assist.8
Patient Preferences and Expectations
Although rigorous techniques are frequently used to evaluate survival and response, less rigor is often used when the impact of treatment on quality of life is assessed. Similar to our case, many patients with lung cancer are elderly with complex medical histories and multiple comorbidities. Given limited survival expectations, symptom palliation, quality of life, and convenience of therapy are especially important end points. This patient wished to be minimally involved in treatment decisions, deferring entirely to his family and the cancer care team. He expressed only his wish to not suffer from “chemo” like his brother had a few years previously. Other patients prefer that family members be excluded from treatment decisions and want to take charge themselves. Becoming actively involved in one’s own cancer treatment may actually improve care and recovery after treatment. For instance, when patients are made fully aware of the potential side effects of treatment, they can promptly alert their cancer care team in case of problems.8
Procedural Strategies
Indications
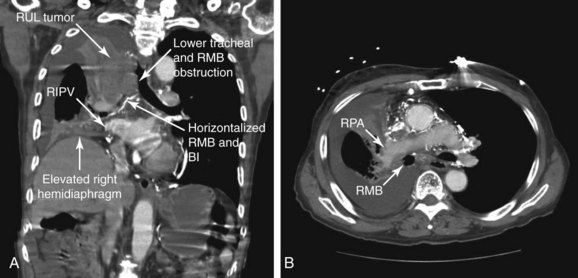
The gold standard treatment for malignant CAO is surgical resection. Similar to this case, however, many patients are poor surgical candidates on the basis of their physiology or oncologic criteria (e.g., inoperable because of advanced tumor stage). Interventional bronchoscopic procedures, when indicated in patients with inoperable NSCLC or SCLC, are expected to restore airway patency and improve lung function, symptoms, and functional status, thus allowing initiation of systemic therapy, which might improve survival.9,10 Resectional techniques are used in cases of intraluminal disease. Airway stent insertion is performed in the setting of malignant CAO caused by severe extrinsic compression (Figure 28-2), or when more than 50% obstruction is present after debulking of the intraluminal component of the disease.11
Contraindications
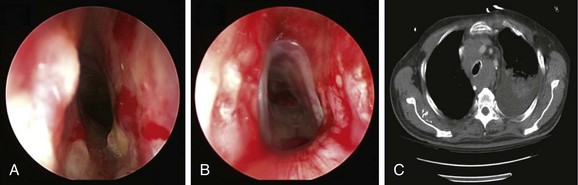
No absolute contraindications to rigid bronchoscopy were noted. Because complete obstruction of the right upper lobe (RUL) bronchus occurred with no identifiable lumen and a potentially nonfunctioning distal lung parenchyma* (Figure 28-3), bronchoscopic attempts to restore RUL bronchial patency were contraindicated. SVC syndrome was a relative contraindication given concerns for hemodynamic instability.
Expected Results
This patient had complete obstruction of the right main bronchus and severe (70%) obstruction in the distal trachea. Palliating CAO was expected to improve dyspnea, lung function, and quality of life.9,12,13 In patients with inoperable or recurrent NSCLC or SCLC occluding a central airway, studies showed no difference in overall survival between those who received both neodymium-doped yttrium aluminum garnet (Nd:YAG) laser treatment and radiation therapy (mean external dose, 53.1 Gy) as compared with historical controls treated with radiation therapy alone. In patients with restored airway lumen,† however, the time interval from treatment to death was prolonged by 4 months compared with those for whom a fully patent airway could not be restored.14 Successful restoration of patency of a major airway occluded by intraluminal tumor using laser resection reduces the likelihood of respiratory failure as a cause of death and does not affect the likelihood of massive fatal hemorrhage, which was a major cause of death in these patients with or without laser treatment.14 Therefore a major issue in these patients is whether they and their families should be warned of the possibility of major bleeding and informed of measures to take should this unfortunate event occur outside the hospital setting.*
Team Experience
Maintaining hemodynamic and respiratory stability during anesthesia requires constant communication between the bronchoscopist and the anesthesiologist. Procedures may be complicated by frequent periods of apnea, compromised airway seals, and the need for special ventilatory techniques such as spontaneous-assisted ventilation or high-frequency jet ventilation. Rigid bronchoscopic procedures involve repeated alternating periods of high and low stimulation requiring rapid titration of intravenous anesthetics to meet fluctuating demands.15
Risk-Benefit Analysis
Because the goal of the procedure is palliation, treatment should have the least possible risk of side effects and discomfort. The risks of intervention warrant careful consideration when patients have significant comorbidities such as a large mediastinal mass, SVC syndrome, or very advanced age.16,17 In our patient, the risks of further physiologic compromise, massive bleeding, and hemodynamic instability were considered to be outweighed by the potential benefit of restoring airway patency to improve functional status and offer systemic therapy. Therapeutic strategies should be elaborated on a case-by-case basis, and advantages and disadvantages of various alternatives discussed with the patient and family if desired, before or during the informed consent process.
Therapeutic Alternatives for Restoring Airway Patency
1. Emergent external beam radiation therapy (EBRT) could have been used for palliating the airway obstruction and the SVC syndrome.18 Initiation of EBRT as primary treatment without attempts at restoration of airway patency in this patient in our opinion would have been of doubtful benefit. EBRT is only variably effective for cancer-induced CAO when the obstruction is severe enough to cause atelectasis, as occurred in our patient. In a study of 330 patients, EBRT palliated hemoptysis in 84% of patients and SVC syndrome in 86% of patients, but atelectasis in only 23%.3 However, EBRT following effective laser treatment could potentially improve survival.19 EBRT had not been recommended as the primary treatment in our patient because of concerns for worsening tracheal obstruction by radiation-induced edema and because of ongoing sepsis in the setting of post obstructive pneumonia. It is noteworthy, from a systemic therapy perspective, that a meta-analysis showed no obvious benefit for combined chemotherapy and radiation therapy over chemotherapy alone in limited-stage SCLC patients older than 70 years. However, more recent trials have revealed a clear-cut benefit for physically “fit elderly”* patients to receive combined modality therapy versus chemotherapy alone, although outcome generally remains inferior to that of younger patients.20
2. Chemotherapy for SCLC involves cisplatin-etoposide regimens and in general is combined with chest radiotherapy for limited disease.21 A systematic review of 29 trials involving 5530 patients found that platinum-based chemotherapy regimens did not offer a statistically significant survival benefit or overall tumor response compared with non–platinum-based regimens. However, platinum-based chemotherapy regimens did increase complete response rates† at the cost of more frequent adverse events, including nausea and vomiting, anemia, and thrombocytopenia.22 CAO is present in as many as 20% to 30% of patients with lung cancer. These patients may develop post obstructive pneumonia.23 Acute infection, as was seen in our patient, is a contraindication to administration of chemotherapy and is an exclusion criterion in most clinical trials.24 We therefore decided to initially proceed with bronchoscopic restoration of airway patency with the patient under general anesthesia.
Techniques and Results
Anesthesia and Perioperative Care
In a patient with severe CAO, premedication with opiates or benzodiazepines in the preoperative period, along with concerns for airway obstruction, anxiety, pain, and periprocedural agitation, should be avoided, or these agents should be administered with caution because they can lead to tachypnea and high airflow velocity during breathing, which increases already turbulent flow in the narrowed airways, exacerbates the pressure drop along the stenosis, and worsens the work of breathing. If possible, patients should be transported to the operating room in a head-up sitting position to avoid worsening preexistent airway and vascular obstruction.25 This simple maneuver can reduce the likelihood of precipitating airway obstruction.26
Careful preoperative assessment for neuromuscular weakness is important in patients with SCLC because approximately 8% of patients with LEMS develop respiratory failure requiring mechanical ventilation. This may develop spontaneously or may be induced by general anesthesia.27 Patients are sensitive to neuromuscular blocking agents and volatile anesthetics, which may cause prolonged paralysis and residual muscle weakness.15 Ideally, these agents should be avoided. Furthermore, the delivery of volatile anesthetics may be problematic when rigid bronchoscopy is performed using an open system with spontaneous-assisted ventilation because the quantity of anesthetic gas reaching the patient is uncertain, and the operating room personnel and operator are at risk for pollution from the escaped anesthetics.15 Total intravenous anesthesia (TIVA) is a preferred technique for rigid bronchoscopy using an open system.*
Securing the airway by rigid bronchoscopic intubation in patients with a large mediastinal mass associated with SVC syndrome and CAO can be challenging. In patients with SVC syndrome, airway management can be complicated by upper airway edema, hemodynamic instability during general anesthesia, and procedure-related bleeding.28 The same degree of edema that is seen externally in the face and neck can be present in the mouth, oropharynx, hypopharynx, and larynx.29 Prompt and atraumatic insertion of the rigid bronchoscope prevents further worsening of preexisting upper airway edema related to severe SVC obstruction. By standing at the head of the patient’s bed during induction, the bronchoscopist is ready and equipped to ensure airway control and, if necessary, to bypass the obstruction, especially in cases of tracheal lesions.30
Anesthesia in the supine position can lead to a decrease in the dimensions of the thoracic cage, a cephalad displacement of the dome of the diaphragm, and a reduction in thoracic volume. Although patients may be asymptomatic while awake, they may develop critical airway obstruction during anesthesia as the result of reduction in the dimensions of the chest wall. This limits the available space for the airways relative to the tumor and mediastinal structures. The decrease in tracheal distention pressure caused by the action of anesthetic agents on chest wall muscle tone also promotes central airway collapse,26 and the supine position prompts an increase in central blood volume, which further increases tumor blood volume and size, worsening both the SVC syndrome and the CAO. Positional changes should certainly be initiated if inadequate ventilation is evident, because airway obstruction sometimes might be ameliorated by placing patients into a lateral decubitus or sitting position.
Although TIVA is used routinely in our institution, loss of airway control has been reported during induction using both intravenous and inhalation anesthesia for rigid bronchoscopy.26,30,31 Muscle relaxants or a dose of propofol that produces apnea can be disastrous in a patient who cannot be ventilated because of CAO.17 Anesthesia induction is the most dangerous period in terms of cardiovascular instability when drugs with a marked tendency for hemodynamic depression are used. Careful hemodynamic monitoring should be provided because patients with SVC syndrome are prone to decreased venous return, reduced cardiac output, and refractory hypotension. Acute worsening of symptoms has been reported as a result of overly generous fluid administration, prompting some authors to recommend diuresis in patients with clinically overt findings of SVC syndrome. It is assumed that diuresis will also decrease tumor volume,25 but diuresis can decrease cardiac preload leading to hypotension, worsening a situation already complicated by compromised venous return.
Spontaneous-assisted ventilation* was shown to have a good cardiac safety profile in patients undergoing rigid bronchoscopy under general anesthesia.31 Although a deeper level of anesthesia is required occasionally to avoid excessive cough and bucking secondary to bronchoscope-induced stimuli, light anesthesia allows spontaneous ventilation. This helps maintain hemodynamic stability and improve oxygenation. Again, we prefer to avoid using neuromuscular blocking agents because they eliminate airway muscular tone that helps maintain airway patency, which may result in prolonged weakness leading to postoperative respiratory failure.30 Positive-pressure ventilation will increase the flow velocity and promote turbulent flow past the region of stenosis. Subsequently, the laminar flow pattern cannot be resumed, potentially resulting in ineffective ventilation of the distal airways and loss of effective gas exchange.29
Ideally, we like to see our patients waking up on the table, avoiding any need for postprocedural endotracheal intubation. During anesthesia maintenance, continuous positive-pressure ventilation and deep anesthesia are also avoided to preserve a normal transpulmonary pressure gradient and to maintain airway patency during spontaneous inspiration.17 This may result in parts of the procedure being performed in a slightly moving or even an occasionally bucking patient. Although precautions are warranted to avoid airway trauma or excessive bronchoscope-related stimulation, short maneuvers are possible, and brief patient movements do not always warrant additional anesthetic to make the patient immobile.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree