INDICATIONS/CONTRAINDICATIONS
General indications for segmentectomy include indeterminate solitary pulmonary nodules, limited infectious or inflammatory disease and selected early stage nonsmall cell lung cancer (NSCLC). Segmentectomy should not be performed in patients who do not fulfill standard criteria for conventional lung resection. Relative contraindications include the following: Coagulopathy, skin infection over the site, diffuse lung disease, and extensive metastases.
In 1995, the Lung Cancer Study Group performed a randomized controlled trial demonstrating that sublobar resections for NSCLC smaller than 3 cm resulted in increased rate of locoregional recurrences compared with lobectomy (8.6% vs. 2.2%, respectively). Subsequently, segmentectomy has generally been restricted to patients with marginal cardiopulmonary function. However, proponents of segmentectomy have maintained that the results of this trial were inconclusive because a third of all patients underwent nonanatomic resections, and the trial included patients with tumors up to 3 cm. In several large, nonrandomized studies from the United States, Japan, and Europe, anatomic segmental resection techniques have been shown to achieve comparable rates of recurrence and survival for stage IA NSCLC.
It has been suggested that segmentectomy, in addition to achieving a complete (R0) resection with adequate surgical margins and systematic nodal staging, may be associated with reduced morbidity and mortality as compared with lobectomy, as it can preserve pulmonary parenchyma and lung function and may be the only feasible surgical option in patients with severe pulmonary impairment that could otherwise not tolerate lobectomy. However, phase III studies are lacking in patients with diffuse emphysema, and some studies have shown better postoperative 3-month functional results with lobectomy versus sublobar resections in patients with poor lung function related to diffuse emphysema. In stage IA NSCLC with diffuse emphysema (ppoVEMS <40%), lobectomy demonstrated a double therapeutic role because of lung reduction effect, especially with upper lobectomies.
Optimal Cases for Segmentectomy
 Well-centered, solitary pulmonary nodule in the targeted segment peripheral and 2 cm or less.
Well-centered, solitary pulmonary nodule in the targeted segment peripheral and 2 cm or less.
 A solitary pulmonary nodule in conjunction with other lesions that need to be resected. During cases of suspected metastases, it is sometimes difficult to distinguish a metastatic lesion from a new lung primarily at the time of frozen section. The use of segmentectomy affords a complete, yet parenchymal-preserving, anatomic resection that may provide definitive management in either circumstance.
A solitary pulmonary nodule in conjunction with other lesions that need to be resected. During cases of suspected metastases, it is sometimes difficult to distinguish a metastatic lesion from a new lung primarily at the time of frozen section. The use of segmentectomy affords a complete, yet parenchymal-preserving, anatomic resection that may provide definitive management in either circumstance.
 Small (<1 cm) ground-glass opacities detected on computed tomography imaging (CT scan) that are frequently associated with early cancers (Noguchi A and B adenocarcinoma) and highly amenable to anatomic segmentectomy. These can be associated with a 5-year survival greater than 90%, and a 6-year survival rate of 100% when associated with frozen section.
Small (<1 cm) ground-glass opacities detected on computed tomography imaging (CT scan) that are frequently associated with early cancers (Noguchi A and B adenocarcinoma) and highly amenable to anatomic segmentectomy. These can be associated with a 5-year survival greater than 90%, and a 6-year survival rate of 100% when associated with frozen section.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
General: The preoperative workup for patients undergoing anatomic segmentectomy for a stage I NSCLC should mirror that of patients undergoing conventional lobectomy. Lung cancer staging typically includes a clinical history, physical examination, standard blood work, CT scan, as well as 18F-fluoro-D-glucose positron emission tomography (PET/CT) imaging. Suggestion of N2 (usually 2R, 4R, 7 stations) nodal disease on a CT imaging or PET/CT (short axis >1 cm and uptake SUVmax >2.5, respectively) needs histologic confirmation using endobronchial ultrasound (EBUS) techniques or mediastinoscopy. Suggestion of N1 (10R, 11R, 12R stations) disease may offer guidance for the highest yield targets to be assessed by frozen section during the dissection allowing for reconsideration of lung-sparing strategy.
A dedicated chest CT scan with intravenous contrast of “topographic quality” (high resolution) is critical to determine lesion size, segment location (regarding intersegmental planes and fissure), and to properly identify anatomic structures including intersegmental veins that need to be preserved. We found a CT with 3D reconstruction particularly helpful in performing a safer dissection of pulmonary artery (PA) branches especially when the fissure is fused, by ascertaining the number and direction of right superior segmental arteries (diameter >2 mm). This technique has been reported to identify 95% of PA branches in patients undergoing segmentectomy. An accurate preoperative marking (injection of radiotracer, hook wire, coil markers, radiopaque markers) during CT imaging may be indicated in indeterminate impalpable solitary pulmonary nodules (i.e., ground-glass opacities) to help intraoperative decision regarding the segment to resect.
Bronchoscopy: The most consistent landmarks of upper right lobe segmental anatomy are segmental bronchi that should be accurately identified during preoperative bronchoscopy to rule out anatomical or endobronchial abnormalities.
Lung function: Resection is functionally possible in patients belonging to “good risk” group with predictive postoperative FEV1 >40% of the predicted value (calculated on the basis of spirometry and isotopic scanning), with no major hypoxemia (<60 mm Hg) or hypercapnia (>46 mm Hg). In “high-risk patients” segmentectomy is considered functionally possible when VO2 max during exercise is >10 mL/kg/min.
 SURGERY
SURGERY
Surgical anatomy: (Fig. 21.1) The right upper lobe includes three segments: Superior (apical) S1, posterior S2, and anterior (ventral) S3. Theoretically, it is possible to achieve segmentectomies of S1, S2, and S3. However, ventral segmentectomy is typically not performed because of its technical complexity (challenging exposure of anterior bronchus (SB3) and ventral artery (SA3) located behind the apical vein (SV1)) and difficulty with preservation of the anterior vein (SV3). In case of a stage 1 NSCLC of S1 or S2, bisegmentectomy S1 + S2 are most often performed together to achieve sufficient disease-free margins.
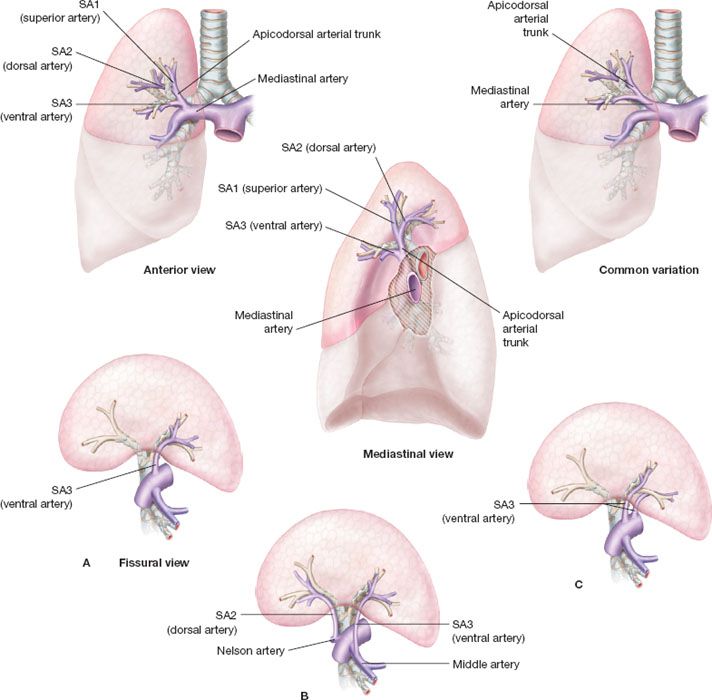
Figure 21.1 Basic anatomy of right superior segments. Upper images: Anterior view; mediastinal view; common variation; mediastinal artery; apicodorsal arterial trunk; SA1 (superior artery); SA2 (dorsal artery); SA3 (ventral artery) arising from mediastinum. Lower images: Different examples of fissural artery (fissural view); A: SA3 (single ventral artery). B: Unusual origin of SA2 and SA3 arising from pulmonary artery or middle lobe artery respectively. C: 2 type of ventraal artery arising from mediastinum or fissura.
Veins: The upper root of the right superior pulmonary vein is made of the superior (SV1), posterior (SV2), and anterior segmental veins (SV3) union. The superior and the posterior vein may receive collaterals from the inferior and middle lobe veins. SV1 is easily recognized and controlled and SV2 is sometimes controllable in the fissure. Usually, veins should be carefully spared during bronchial and arterial dissection and divided as the last step during intersegmental plane management.
Arteries: SA1 and SA3 arise from truncus anterior branch of the PA. The mediastinal artery typically divides into two or three branches: Superior artery (SA1) and anterior artery (SA3). S2 is usually vascularized by SA2, arising from the intralobar PA. SA2 is located at the posterior part of the PA and ascends in front of the right superior lobar bronchus. In some patients, SA2 originates from SA6 (10%) and occasionally can be mistaken for SA3 (when SA3 also come off the intralobar PA).
Bronchus: When it enters lung parenchyma, right upper lobe bronchus divided into three segmental bronchi, SB1 (superior or apical bronchus), SB2 (posterior or dorsal bronchus), and SB3 (anterior or ventral bronchus). SB2 and SB3 may originate from a common “apicodorsal,” “apicoventral,” or “dorsoventral” bronchial trunk or originate separately and thus may be stapled together or separately depending on the required resection.
Positioning and approach: All procedures are performed under general anesthesia and epidural or paravertebral catheters should be available to all patients. A double-lumen endotracheal tube is preferred to a bronchial blocker to enable intraoperative bronchoscopy during one-lung ventilation. The patient is positioned in the lateral decubitus with table slight flexion at the level of scapula tip improving access and exposure. The chest is entered through a standard muscle-sparing posterolateral or anterior thoracotomy in the fourth or fifth intercostal space according to associated lesions and local anatomy. A 5-cm thoracotomy combined with a thoracoscope placed through an anterior single port may be a feasible alternative. The thoracic cavity should be inspected to rule out signs of unexpected advanced disease such as mediastinal node positivity or pleural involvement, and the tumor palpated to reaffirm the planned segmental resection.
Technique
General consideration: We use a peanut dissector, scissors, and suction tip to facilitate gentle distal dissection of segmental pedicle. Precise dissection of fissures requiring punctate electrocoagulation and isolated application of ligatures can be conducted with the help of magnifying loupes. Control of large vessels is accomplished with vascular staplers or nonabsorbable 3/0 to 4/0 ligatures while hemostasis of small caliber vessels is accomplished with clips or a bipolar vessel-sealing device (LigaSure, Valleylab, Boulder, CO, USA).
Surgical steps:
1. (Fig. 21.2). After opening the perihilar mediastinal pleura and removing the 10R lymph node that lies between the right main bronchus and the azygos, we usually perform an extended distal exposure of the truncus anterior artery. Because the arterial supply to the right upper lobe has several variations, the origins of SA2 and SA6 should be identified in the posterior part of interlobar fissure, and the middle lobe artery, truncus anterior artery with SA1/SA3 origins should be fully exposed anteriorly. We achieve full exposure of the upper lobe pedicle and assess bronchial direction before any arterial division.
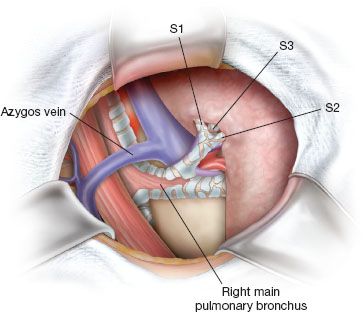
Figure 21.2 Bronchial steps. Perihilar mediastinal pleura has been opened and 10R has been removed. Right superior lobe is gently pulled anteriorly and distal full exposure of SB1 and SB2 is generally easier after dividing posterior part of fissure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


