Dronedarone is a multi-channel-blocking drug for the treatment of patients with atrial fibrillation (AF) or atrial flutter (AFL) with rate- and rhythm-controlling properties. A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d. for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients With Atrial Fibrillation/Atrial Flutter (ATHENA) demonstrated that dronedarone reduced the risk for first cardiovascular hospitalization or death from any cause. The aim of this post hoc analysis was to evaluate the rhythm- and rate-controlling properties of dronedarone in the ATHENA trial. Patients were randomized to dronedarone 400 mg twice daily (n = 2,301) or placebo (n = 2,327). Electrocardiographic tracings were classified for AF or AFL or sinus rhythm. Patients with AF or AFL on every postbaseline electrocardiogram were classified as having permanent AF or AFL. All electrical cardioversions were documented. The use of rate-controlling medications was equally distributed in the 2 treatment groups. The median time to first AF or AFL recurrence of patients in sinus rhythm at baseline was 498 days in placebo patients and 737 days in dronedarone patients (hazard ratio 0.749, 95% confidence interval 0.681 to 0.824, p <0.001). In the dronedarone group, 339 patients (15%) had ≥1 electrical cardioversion, compared to 481 (21%) in the placebo group (hazard ratio 0.684, 95% confidence interval 0.596 to 0.786, p <0.001). The likelihood of permanent AF or AFL was lower with dronedarone (178 patients [7.6%]) compared to placebo (295 patients [12.8%]) (p <0.001). At the time of first AF or AFL recurrence, the mean heart rates were 85.3 and 95.5 beats/min in the dronedarone and placebo groups, respectively (p <0.001). In conclusion, dronedarone demonstrated both rhythm- and rate-controlling properties in ATHENA. These effects are likely to contribute to the reduction of important clinical outcomes observed in this trial.
A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d. for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients With Atrial Fibrillation/Atrial Flutter (ATHENA) demonstrated that dronedarone reduced major clinical outcomes in patients with atrial fibrillation (AF) or atrial flutter (AFL), including cardiovascular hospitalizations, cardiovascular mortality, and stroke. The incidence of adverse events overall in ATHENA was similar in the dronedarone and placebo groups (72% vs 69%, p = 0.048), with no increase in rates of thyroid and pulmonary disorders with dronedarone. The present post hoc analysis was undertaken to examine the rhythm- and rate-controlling effects of dronedarone in ATHENA.
Methods
Details of the study protocol have been published previously. In brief, ATHENA was a randomized, double-blind, placebo-controlled trial conducted in 4,628 patients recruited by 551 centers in 37 countries.
Patients with paroxysmal or persistent AF or AFL and additional cardiovascular risk factors were eligible. For each patient, a 12-lead electrocardiogram (ECG) <6 months before randomization had to be available showing AF or AFL, and a second 12-lead ECG within the same period had to show sinus rhythm. Patients could be included either in sinus rhythm or in AF or AFL. Randomization was stratified according to rhythm status at baseline.
Baseline characteristics and baseline drug therapy were well balanced between the treatment groups. All medications were managed by the enrolling physician. The use of class I or III agents was not allowed during the study, and decisions about cardioversion or dosing of rate-controlling agents, such as β-adrenergic blockers, nondihydropyridine calcium-channel blockers, and digoxin, were at the discretion of the treating physician. Details of the dosing of rate-controlling agents were not recorded in the case report forms.
The follow-up visit schedule included clinical evaluations at days 7 and 14 and at months 1, 3, 6, 9, and 12 and every 3 months thereafter. In case of clinical deterioration, patients were seen as clinically appropriate.
During the course of the study, 12-lead ECGs were recorded at each visit up to 6 months and every 6 months thereafter. The investigator classified each ECG as demonstrating AF or AFL or sinus rhythm, and the ventricular rate was recorded. In addition, ECGs were recorded on other occasions, such as recurrence of symptoms. These scheduled and unscheduled electrocardiographic studies provided the timing of recurrence of AF or AFL for those patients who were in sinus rhythm at the onset of the trial.
Decisions of whether or when to perform electrical cardioversion were at the discretion of the treating physician. All electrical cardioversions were prospectively documented.
All hospitalizations during the study were reported in specific case record forms. The main reason for each cardiovascular hospitalization was classified by the investigator according to a prespecified list of reasons, including AF or AFL.
For the analysis of maintenance of sinus rhythm, only the subpopulation of patients who were in sinus rhythm at study initiation was analyzed. One of the following events was regarded as evidence for recurrence of AF or AFL: AF or AFL on electrocardiography, cardioversion, or hospitalization for AF or AFL, whichever came first. For the purpose of this study, “permanent AF” was defined if every ECG from beginning to end of the study showed AF or AFL.
Analyses were performed on the intention-to-treat population. The time to event was estimated according to the Kaplan-Meier method and compared using the log-rank test. All p values were 2 tailed. Mean heart rates were compared using Student’s t test.
Results
The baseline characteristics were reported in detail previously. Of note, class I and III antiarrhythmic drugs (including sotalol) were contraindicated during the treatment with the study drug. Heart rate control was provided according to the investigator’s judgment; 82% of the placebo patients and 83% of the dronedarone patients received ≥1 rate-controlling agent (β blocker, calcium antagonist with heart rate–lowering effects, or digitalis) at baseline, while 90% and 88% (respectively) received rate-controlling agents during the study.
The antiarrhythmic effect was evaluated in several ways, including time to recurrence of AF or AFL (for the patients in sinus rhythm at baseline) and the time to first cardioversion.
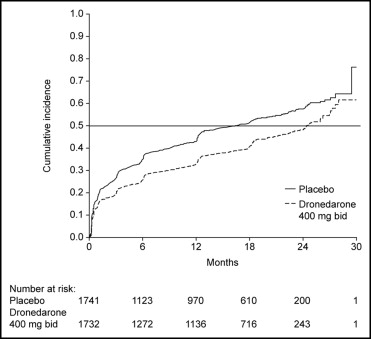
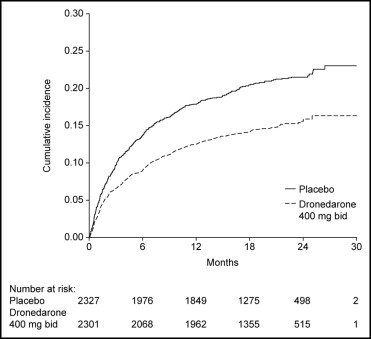
Of the patients enrolled, 3,473 (75%) were in sinus rhythm at baseline as per stratification factor. As shown in Figure 1 , over a mean follow-up period of 21 months, there was a reduction in the likelihood of developing AF or AFL in the dronedarone group (hazard ratio 0.749, 95% confidence interval 0.681 to 0.824, p <0.001). The median time to first recurrence of AF or AFL was prolonged from 498 days with placebo to 737 days with dronedarone. Most of the events were documented as an outpatient on electrocardiography that was either symptom related or part of routine follow-up ( Table 1 ); overall, 96% of the recordings were obtained on the day of scheduled follow-up. Patients receiving dronedarone were less likely to undergo electrical cardioversion (15% [n = 339]) compared to patients receiving placebo (21% [n = 481]) (hazard ratio 0.684, 95% confidence interval 0.596 to 0.786, p <0.001; Figure 2 ).
| Variable | Placebo ⁎ | Dronedarone ⁎ |
|---|---|---|
| (n = 1,741) | (n = 1,732) | |
| Number of patients with event | 950 (55%) | 779 (45%) |
| End point composition | ||
| 12-lead ECG in AF or AFL | 586 (34%) | 525 (30%) |
| Hospitalization for AF or AFL | 265 (15%) | 178 (10%) |
| Electrical cardioversion | 99 (5.7%) | 76 (4.4%) |
⁎ All randomized patients without AF or AFL at baseline as per stratification factor.
Further evidence of the antiarrhythmic effect of dronedarone was suggested by a lower frequency of patients considered in permanent AF or AFL among those receiving dronedarone (n = 178 [7.6%]) compared to those receiving placebo (n = 295 [12.8%]) (p <0.001).
At the time of first recurrence of AF or AFL, the mean heart rate was lower in the dronedarone group (85.3 ± 22.3 beats/min) compared to the placebo group (95.5 ± 25.6 beats/min) (p <0.001; Table 2 ). The effect of dronedarone on ventricular rate control was also evaluated by determining the ventricular rate recorded on all 12-lead ECGs for patients in AF, AFL, and sinus rhythm. Heart rate was lower in all 3 rhythms (AF, AFL, and sinus) in dronedarone-treated patients compared to placebo-treated patients. The median heart rates was 8, 9, and 4 beats/min lower in AF, AFL, and sinus rhythm, respectively. Of note, the administration of other atrioventricular nodal blocking agents was left to the judgment of the treating physician, and dose adjustments were not recorded.



