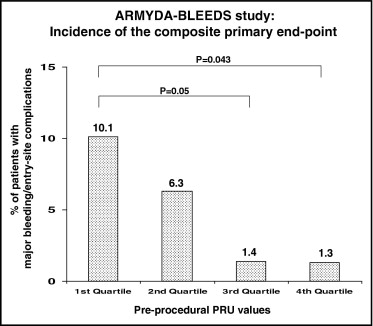
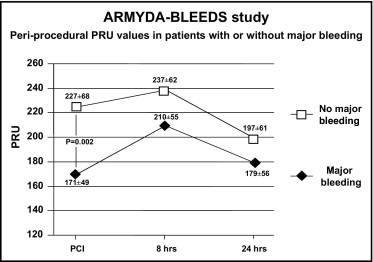
Platelet reactivity predicts ischemic outcomes in patients who undergo percutaneous coronary intervention (PCI), but the correlation of heightened platelet response with bleeding has not been characterized. The aim of this study was to evaluate whether low platelet reactivity by point-of-care measurement after clopidogrel administration correlates with bleeding complications of PCI. A total of 310 patients receiving clopidogrel before PCI were prospectively enrolled. Platelet reactivity was measured with the VerifyNow P2Y12 assay. The primary end point was the 30-day incidence of major bleeding or entry-site complications according to quartile distribution of P2Y12 reaction units (PRU). The primary end point occurred more frequently in patients with preprocedural PRU levels in the lowest quartile compared to those in the highest quartile (10.1% vs 1.3%, p = 0.043), due mainly to entry-site hemorrhages. Absolute PRU levels were lower in patients with major bleeding (171 ± 49 vs 227 ± 68 in patients without, p = 0.002). On multivariate analysis, pre-PCI PRU levels in the first quartile were associated with a 4.5-fold increased risk for major bleeding (odds ratio 4.5, 95% confidence interval 1.9 to 25.9, p = 0.01). By receiver-operating characteristic curve analysis, the optimal cutoff for the primary end point was a pre-PCI PRU value ≤189 (area under the curve 0.76, 95% confidence interval 0.66 to 0.87, p = 0.001). In conclusion, this study suggests that an enhanced response to clopidogrel may be associated with higher risk for early major bleeding or entry-site complications in patients who undergo PCI. Point-of-care monitoring of platelet reactivity after clopidogrel administration may help identify patients in whom individualized strategies are indicated to limit bleeding complications after coronary intervention.
Significant interindividual variability in clopidogrel response has been demonstrated, and various investigations correlated low clopidogrel responsiveness with enhanced risk for early and late adverse cardiac events in the setting of percutaneous coronary intervention (PCI). Conversely, there is a gap of knowledge on the possible relation between higher clopidogrel responsiveness and increased bleeding risk. Because periprocedural bleeding complications strongly impair prognosis after PCI, assays measuring residual platelet reactivity after clopidogrel administration might help rapidly stratify patients according to their bleeding risk. The Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty (ARMYDA) study group has designed the ARMYDA–Bleeding Study (ARMYDA-BLEEDS) to prospectively evaluate levels of platelet reactivity by a point-of-care assay that may correlate with bleeding complications in patients who undergo coronary stent implantation.
Methods
ARMYDA-BLEEDS is a prospective study of 310 consecutive clopidogrel-treated patients who underwent PCI at Campus Bio-Medico University of Rome from April 1 to December 31, 2009. This population represents 69% of patients (310 of 449) who underwent PCI at our institution during the enrollment period. Inclusion criteria were (1) indication for percutaneous coronary revascularization for angina with inducible myocardial ischemia or non–ST-segment elevation acute coronary syndromes (ACS) and (2) clopidogrel therapy initiated before PCI, as a 600-mg load given ≥6 hours before intervention (n = 104) or long-term clopidogrel therapy 75 mg/day for ≥5 days (n = 206). Exclusion criteria were indication for long-term therapy with vitamin K antagonists, intervention for ST-segment elevation acute myocardial infarction <24 hours (representing most patients excluded from the study from the whole cohort of PCI patients during the enrollment period), platelet count <70 × 10 9 /L, coronary bypass surgery in the previous 3 months, and severe chronic renal failure with baseline serum creatinine >2 mg/dl. All interventions were performed using the femoral approach, with weight-adjusted intravenous unfractionated heparin (70 IU/kg body weight). During PCI, bivalirudin was used instead of unfractionated heparin in patients considered at high bleeding risk (age >75 years, history of previous bleeding, renal failure, low body weight); periprocedural use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion according to the presence of thrombus at the site of the index stenosis, occurrence of no-reflow, or vessel closure. The sheath size was 6Fr (7Fr in case of bifurcating lesions). The arterial sheath was removed when the activated clotting time was <180 seconds. The femoral arteries were closed by manual compression in all cases; manual compression was performed until the achievement of full hemostasis plus an additional 5 minutes. By protocol, patients receiving long-term clopidogrel therapy were not reloaded in the catheterization laboratory. Clopidogrel was continued (75 mg/day) for 1 month after PCI, except in patients receiving drug-eluting stents or treated for ACS, in whom the drug was discontinued 1 year after intervention. Aspirin was given to all patients and continued indefinitely.
Platelet reactivity was evaluated in the catheterization laboratory immediately before PCI and at 8 and 24 hours after intervention using the VerifyNow P2Y12 assay (Accumetrics, Inc., San Diego, California), a rapid cartridge-based assay specifically measuring the direct effects of clopidogrel on the platelet P2Y12 receptor. Technical details have been described elsewhere. Results are expressed as P2Y12 reaction units (PRU), which inversely correlate with the degree of P2Y12 receptor inhibition by clopidogrel. Twenty-five randomly selected patients were analyzed to assess intra-assay variability, which was 2.0 ± 1.1% (coefficient of variation 6%). Blood samples were also drawn before and at 8 and 24 hours in all patients to measure hemoglobin levels; further hemoglobin determinations were performed if clinically indicated. Patients were clinically evaluated after PCI for the detection of bleeding events and entry-site complications (hematoma, pseudoaneurysm, or arteriovenous fistula). One-month clinical follow-up was then obtained by office visit in all patients. Each patient gave informed consent to the study.
The primary end point of ARMYDA-BLEEDS was the 30-day incidence of major bleeding or significant entry-site complications in relation to periprocedural quartile distribution of platelet reactivity measured by PRU assay. Major bleeding was defined according to the Thrombolysis In Myocardial Infarction criteria. Significant entry-site complications were defined as hematoma >10 cm in diameter, pseudoaneurysm, or arteriovenous fistula. Secondary end points were (1) evaluation of absolute PRU values in patients with or without major bleeding and (2) correlation of PRU values with minor bleeding or post-PCI hematoma ≤10 cm in diameter.
Continuous variables were compared using Student’s t tests for normally distributed values; otherwise, Mann-Whitney U tests were used. Proportions were compared using Fisher’s exact test when the expected frequency was <5; otherwise, chi-square tests were applied. Odds ratios and 95% confidence intervals investigating the independent predictive role of PRU quartiles on the occurrence of the primary end point were assessed by logistic regression. The following parameters were first evaluated in a univariate model: PRU quartile, age, gender, body mass index, diabetes mellitus, clinical presentation (stable angina vs ACS), chronic renal failure, hemoglobin levels, previous transient ischemic attack or stroke, previous major bleeding, use of bivalirudin versus unfractionated heparin, and use of glycoprotein IIb/IIIa inhibitors. Variables with p values <0.15 were then entered into the final model of multivariate logistic regression analysis. The ability of the assay to discriminate between patients with and without major bleeding at 30 days was evaluated using receiver-operating characteristic curve analysis. The optimal cut-off value was calculated by determining the PRU value providing the greatest sum of sensitivity and specificity. Results are expressed as mean ± SD. Two-tailed p values <0.05 were considered significant. Analysis was performed using SPSS version 12.0 (SPSS, Inc., Chicago, Illinois).
Results
Clinical and procedural characteristics according to preintervention PRU quartiles are listed in Table 1 . Procedural success was achieved in 98% of patients, without need for repeat PCI or coronary artery bypass grafting at 30 days.
| Characteristic | First Quartile | Second Quartile | Third Quartile | Fourth Quartile | p Value |
|---|---|---|---|---|---|
| (n = 77) | (n = 77) | (n = 77) | (n = 79) | ||
| Age (years) | 65 ± 9 | 65 ± 10 | 68 ± 11 | 68 ± 9 | 0.07 |
| Women | 16 (21%) | 12 (16%) | 19 (25%) | 20 (25%) | 0.59 |
| Diabetes mellitus | 30 (39%) | 26 (34%) | 25 (32%) | 34 (43%) | 0.68 |
| Hypercholesterolemia (>200 mg/dl) | 55 (71%) | 51 (66%) | 52 (68%) | 62 (79%) | 0.44 |
| Body mass index (kg/m 2 ) | 28 ± 4 | 28 ± 6 | 27 ± 5 | 28 ± 4 | 0.49 |
| Previous myocardial infarction | 20 (26%) | 26 (34%) | 24 (31%) | 21 (27%) | 0.92 |
| Previous transient ischemic attack/stroke | 2 (3%) | 2 (3%) | 4 (5%) | 2 (3%) | 1 |
| Previous PCI | 34 (44%) | 33 (43%) | 25 (32%) | 31 (39%) | 0.61 |
| Previous major bleeding | 2 (3%) | 1 (1%) | — | 2 (3%) | 0.73 |
| Clinical presentation | |||||
| Unstable angina/non–ST-segment elevation myocardial infarction | 19 (25%) | 26 (34%) | 29 (38%) | 26 (33%) | 0.50 |
| Stable angina | 58 (75%) | 51 (66%) | 48 (62%) | 53 (67%) | 0.50 |
| Left ventricular ejection fraction (%) | 54 ± 10 | 55 ± 7 | 54 ± 11 | 57 ± 8 | 0.12 |
| Serum creatinine (mg/dl) | 1.11 ± 0.23 | 1.10 ± 0.21 | 1.12 ± 0.25 | 1.08 ± 0.3 | 0.77 |
| Hemoglobin (g/L) | 13.2 ± 1.78 | 13.5 ± 1.73 | 13.0 ± 1.72 | 13.1 ± 1.33 | 0.27 |
| Vessel treated | |||||
| Left anterior descending coronary artery | 41 (47%) | 47 (51%) | 44 (50%) | 45 (45%) | 1 |
| Left circumflex coronary artery | 18 (20%) | 25 (27%) | 21 (24%) | 25 (25%) | 1 |
| Right coronary artery | 28 (32%) | 20 (22%) | 23 (26%) | 29 (29%) | 0.64 |
| Left main coronary artery | 1 (1%) | — | — | 1 (1%) | 0.80 |
| Lesion type B2/C | 48 (62%) | 56 (73%) | 50 (65%) | 55 (70%) | 0.70 |
| Multivessel intervention | 14 (18%) | 15 (19%) | 14 (18%) | 15 (19%) | 1 |
| Use of drug-eluting stents | 19 (25%) | 23 (30%) | 29 (38%) | 24 (30%) | 0.51 |
| Statin therapy | 64 (83%) | 72 (94%) | 70 (91%) | 74 (94%) | 0.12 |
| Sheath size | |||||
| 6Fr | 72 (94) | 72 (94) | 71 (92) | 73 (92) | 1 |
| 7Fr | 5 (6) | 5 (6) | 6 (8) | 6 (8) | 1 |
| Antithrombotic therapy | |||||
| Unfractionated heparin | 64 (83%) | 68 (88%) | 66 (86%) | 74 (94%) | 0.29 |
| Bivalirudin | 13 (17%) | 9 (12%) | 11 (14%) | 5 (6%) | 0.29 |
| Glycoprotein IIb/IIIa inhibitors | 9 (12%) | 8 (10%) | 11 (14%) | 6 (8%) | 0.83 |
The overall incidence of major bleeding or entry-site complications was 4.8% (15 of 310 patients): 3 patients had gastrointestinal bleeding, 2 had bladder or urethral bleeding, and the remaining had entry-site hematomas >10 cm. Patients in the lowest PRU quartile before PCI had a higher incidence of major bleeding at 1 month (10.1%) compared to those in the highest quartile (1.3%, p = 0.043) and the third quartile (1.4%, p = 0.05) ( Figure 1 ). Absolute PRU values before PCI were lower in patients with compared to those without major bleeding at 30 days (171 ± 49 vs 227 ± 68, p = 0.002). Periprocedural PRU values in patients with or without major bleeding are reported in Figure 2 ; PRU increased at 8 hours after PCI and decreased over 24 hours.