Rheumatic Fever and Rheumatic Heart Disease
Lloyd Y. Tani
Acute rheumatic fever (RF) occurs as a result of a complex interaction between group A streptococcus (GAS), a susceptible host, and the environment. An abnormal immune response to GAS infection leads to an acute inflammatory illness that most commonly affects the joints, brain, heart, and/or skin. Although the other manifestations are self-limiting and resolve without sequelae, carditis may result in chronic rheumatic heart disease (RHD) with associated significant morbidity and mortality. The degree of cardiac involvement is quite variable, ranging from very mild, subclinical valvulitis to severe carditis with significant acute mitral and/or aortic regurgitation resulting in heart failure. The acute rheumatic cardiac involvement may resolve or persist and evolve as chronic rheumatic valvular disease, with cardiac symptoms developing years after the initial episode.
Epidemiology
Scope of the Problem
RF continues to be a major public health problem in developing countries, where over 80% of children younger than 15 years of age live, and where it is the most common cause of acquired cardiac disease in children and young adults (1,2,3,4,5,6,7). Worldwide, it is estimated that at least 470,000 cases of RF occur annually in patients of all ages, with approximately 340,000 cases occurring in children 5 to 14 years of age. The majority of cases occur in developing countries and in indigenous populations, where the reported incidence is as high as 200 to 300 per 100,000 (3,4,8,9). Because of the difficulty in obtaining data in these regions and populations, it is possible that the true incidence in some areas is even higher; community-based surveillance suggests that the true incidence in some settings may be as high as 500/100,000 (10,11). In these regions, the current situation is similar to that experienced by developed countries in the early part of the 20th century.
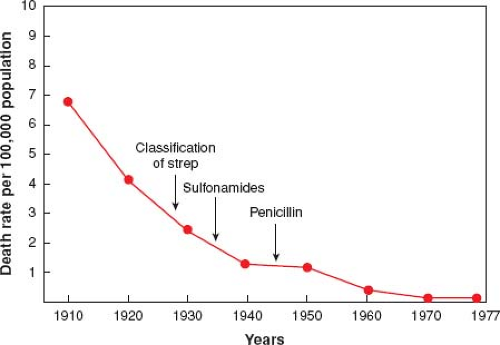
In sharp contrast, there has been a significant decline in the incidence of RF and RHD over the last 50 years in most developed countries of the world (Fig. 59.1). The initial decline, which began prior to the initiation of penicillin, was at least partly due to improved socioeconomic conditions. With the subsequent initiation of penicillin, there was further acceleration in the rate of decline of RF (12,13). Changes in streptococcal strains and decreased virulence may have also contributed to the decline in RF seen in these countries (14). These changes have resulted in a marked decline in mortality due to acute rheumatic carditis from 8% to 30% to nearly zero (12,13). In fact, the decrease in the incidence of RF in developed countries was so dramatic that the disease was thought to have “virtually disappeared” by the early 1980s (13).
In the mid-1980s, several sites in the United States reported focal outbreaks or resurgences of RF activity (15,16,17,18,19,20), and by 1988, a survey of pediatric cardiologists revealed a 5 to 12 times increased incidence of RF in 24 states (21). Unique features of this resurgence included: (1) many cases came from suburban/rural neighborhoods; (2) the majority of patients were Caucasian and from middle class families with medical insurance and ready access to medical care; (3) there was no clear-cut evidence of crowding; (4) a preceding sore throat prompting the patient and family to seek medical attention was relatively uncommon. Although the reason(s) for these resurgences is unclear, the appearance of certain strains of GAS (in particular heavily encapsulated or mucoid strains) in the
community coincident with the reported resurgence in Utah supports the concept of “rheumatogenicity” (22). Despite these resurgences, the incidence of RF in developed countries is much lower than in developing countries, and has been estimated at 0.5 to 3 per 100,000 population (5,13,23).
community coincident with the reported resurgence in Utah supports the concept of “rheumatogenicity” (22). Despite these resurgences, the incidence of RF in developed countries is much lower than in developing countries, and has been estimated at 0.5 to 3 per 100,000 population (5,13,23).
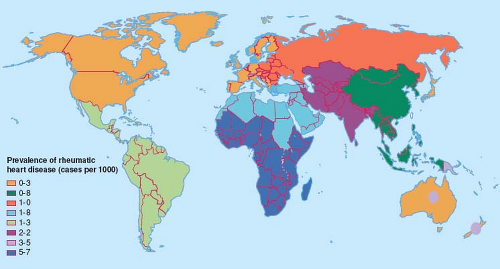
The prevalence of RHD parallels the reported incidences of RF. Both RF and RHD continue essentially unabated in many developing countries of the world, where RHD remains an important and significant cause of morbidity and mortality (Fig. 59.2). Worldwide, it is estimated that 15 to 20 million people have RHD (5), of whom about 2.4 million are children between the ages of 5 and 14 years (9). Given the current estimates of RF incidence and the proportion of patients who develop RHD, it is estimated that at least 282,000 people develop RHD each year (9). The prevalence of RHD varies greatly between countries, ranging from <0.5/1,000 in developed countries of the world (9) to 78/1,000 in Samoa (24), and increases with age, peaking in young adults aged 25 to 34 years (11). The older prevalence data based on clinically detected RHD may significantly underestimate the true disease burden. Recent studies using echocardiography reported prevalences of the order of 10-fold greater than that detected clinically, suggesting that ∼90% of RHD cases are subclinical (see the section below on screening for RHD) (25,26).
Compared to cases occurring in industrialized countries, the initial episode of RF in developing countries occurs at a younger age, and often goes unnoticed. In these settings, many, if not most, of the affected individuals are unaware of their disease and therefore do not receive secondary prophylaxis (4,27). Compared to the natural history in developed countries, chronic RHD in developing countries occurs earlier, evolves more rapidly, and is of greater severity, more commonly leading to heart failure with its associated morbidity and mortality (28,29,30,31,32,33,34,35,36). Once significant RHD has developed, the means of caring for such patients is also limited in many of these developing countries. In 1994, it was estimated that at least 3 million people with RHD required repeated hospitalization for heart failure (4). In developing countries, a significant proportion of all cardiac admissions are for RHD, with an average length of stay of 3 to 4 weeks, resulting in absence from work and reduced productivity (37). In 2001, an estimated 6.6 million disability-adjusted years were lost to RHD. The mortality rate from RHD has been estimated to range from 0.9 to 8.0 per 100,000 population (4,5,38,39). Based on conservative estimates, a minimum of 1.5% of patients with RHD die each year in developing countries where secondary prophylaxis is uncommonly given and medical and surgical treatments are often unavailable. Worldwide, it has been estimated that 233,000 to 492,000 deaths per year occur due to RHD, with 95% of the mortality occurring in developing countries (9). Further, patients with RHD are at increased risk for endocarditis and stroke, complications associated with additional morbidity and mortality (9,27,34). In contrast, an analysis of more than 2.5 million hospital discharges in 2000 from more than 2,784 institutions in 27 states in the United States reported only 503 hospitalizations for RF among children <21 years (40). A recent study in the United States reported a significant burden of RHD amongst immigrants from developing countries (41).
Environment
Despite the epidemiologic link between GAS pharyngitis and RF, other factors clearly influence the incidence of RF. Current data suggest that the incidence of GAS pharyngitis has remained more or less stable in most countries, and that there has been no change in host resistance to the GAS organism. Therefore, the marked difference in the incidence of RF and prevalence of RHD in developing versus developed countries is likely due to other factors. The importance of environmental and socioeconomic factors in the epidemiology and pathogenesis of RF has been recognized for decades. In developing countries, overcrowding, poverty, poor nutrition, poor hygiene, and poor access to health care are common and contribute to rapid spread (respiratory droplets) and increased virulence of GAS (42,43). In particular, overcrowding appears to be a major factor contributing to the high incidence of RF in many parts of the world. With poor access to health care, GAS pharyngitis is less
likely to be diagnosed and treated, precluding effective primary prevention of RF. In addition, because cases of RF are more likely to go unnoticed, secondary prophylaxis is not implemented and RF recurrences are common.
likely to be diagnosed and treated, precluding effective primary prevention of RF. In addition, because cases of RF are more likely to go unnoticed, secondary prophylaxis is not implemented and RF recurrences are common.
The seasonal variation of RF in the temperate climates parallels that of GAS pharyngitis. Both GAS pharyngitis and RF are more common during the winter and spring in temperate climates, but there is no consistent seasonal pattern in the tropics. Geographically, RF occurs in all latitudes and altitudes (42).
Host
Children between the ages of 5 and 15 years are most commonly affected. RF is uncommon before age 5 years, almost never occurs before 2 years of age, and is uncommon beyond the age of 35 years (3). Children with RF before age 5 years commonly present with arthritis and rarely present with chorea; when present, cardiac involvement is more severe than in older children and persistent RHD is common (44,45). Adults with a primary episode of RF are much more likely to have joint manifestations than cardiac involvement (46). Recurrences are most frequent during adolescence and early adulthood. With the exception that chorea is more common in girls, there is no definite gender predisposition (47,48,49,50).
There is evidence supporting the importance of a host predisposition to developing RF. First, only a small minority of patients with streptococcal pharyngitis develops RF, even during streptococcal epidemics (∼3%). Second, the incidence of recurrent RF in patients with a previous history of RF with cardiac involvement is as high as 50% following GAS pharyngitis (51,52,53). Third, studies indicate a familial predilection (54,55) and a higher concordance rate between identical twins than in fraternal twins (44% vs. 12%) (56,57,58). Higher rates of RF and RHD have also been reported in certain ethnic groups, specifically Maoris and Pacific Islanders in New Zealand, Samoans in Samoa and Hawaii, and Aboriginal people in Australia (59,60,61,62). (See section on Susceptible Host in the Pathogenesis section.)
Streptococcal Infection
Most children have at least one episode of pharyngitis per year, approximately 10% to 30% of which are due to GAS, the most common bacterial cause of pharyngitis (63,64). Streptococcal pharyngitis occurs most commonly in children aged 5 to 15 years, and is uncommon before the age of 2 years. Although GAS may be present in the pharynx with either true infection and pharyngitis or a carrier state, only true infection results in an immune response and risk for the development of RF (65). The prevalence of GAS carrier state varies considerably (5% to 30%), depending on the population and series (66,67). Approximately 0.3% (during nonepidemic) to 3.0–5.0% (during streptococcal epidemics) of individuals who have not had RF will develop the illness following an untreated symptomatic or asymptomatic streptococcal pharyngitis. Older studies reported a relationship between the clinical severity of the pharyngitis and likelihood of developing RF, but numerous reports emphasize that RF occurs following very mild or asymptomatic pharyngitis in up to two-thirds of cases (15,68,69,70).
The strain and virulence of the streptococcal organism influence the likelihood of development of RF. In the 1930s, GAS strains that reactivated RF were noted to be different from strains that did not (71). Other investigators subsequently found that some strains were associated with pharyngitis while other strains were associated with skin infections (72). Further, certain GAS strains have been associated with RF, while others have been associated with poststreptococcal glomerulonephritis (73). Based on epidemiologic data, certain GAS strains are more likely to lead to RF (“rheumatogenic”) than others (“nonrheumatogenic”) (47,72,74). The M protein is thought to be a major virulence factor because it affects the ability of host cells to undergo phagocytosis. Of the greater than 130 M types, M types 1, 3, 5, 6, 14, 18, 19, 24, 27, and 29 have been associated with outbreaks of RF, while M types 2, 4, 12, 22, and 28 rarely lead to RF (5,14,75,76). Further evidence of the importance of the M protein came from the discovery that epitopes of the M protein molecule cross-react antigenically with human heart and brain tissue. Some have reported an association between the appearance of heavily encapsulated (“mucoid”) strains in a community and an increase in the number of RF cases (22,77,78,79,80,81). Finally, there is evidence that the decrease in the incidence of RF in developed countries is in part due to a change in GAS strains (altered expression of M protein) and a decrease in the incidence of GAS pharyngitis caused by rheumatogenic strains (14).
Despite the evidence for GAS pharyngitis but not impetigo as the initiating event leading to RF, there is some recent epidemiologic evidence to suggest that skin strains may play a role in some populations. In the Aboriginal population of Australia where RF is endemic, GAS impetigo is common but GAS pharyngitis is uncommon. Although direct causation, immune priming, and movement of strains from the skin to the pharynx have been postulated, the precise role of GAS skin infections in the pathogenesis of RF remains to be elucidated (82,83,84).
Pathogenesis
Organism
RF occurs as a result of a complex interaction between the GAS organism, host, and environment. Although the relationship to a preceding streptococcal pharyngitis is established and well accepted, the pathogenesis of RF is still not completely understood. Initially thought to occur as a result of direct injury by the GAS or its toxins, the organism has not been isolated from the organ systems of affected individuals. Current evidence strongly suggests that RF occurs as a delayed autoimmune response to pharyngitis caused by a rheumatogenic strain of GAS in a susceptible individual. Streptococcal antigenic components trigger abnormal autoimmune humoral and cellular responses that lead to the various manifestations of RF. Several lines of evidence led to and support the concept of RF occurring after GAS pharyngitis: (1) documented relationship between GAS pharyngitis epidemics and RF (12,42), (2) immunologic evidence of a preceding streptococcal infection via elevated/rising antibody titers (85), (3) adequate treatment of GAS pharyngitis can prevent RF (86,87), (4) antibiotic prophylaxis can prevent RF recurrences (88,89,90), (5) mass prophylaxis with penicillin terminated RF epidemics in military and civilian populations (91,92,93).
Susceptible Host
Most of the clinical manifestations of RF occur approximately 10 days to 5 weeks (average 18 days) following GAS pharyngitis in a susceptible host (94). A number of sources support the importance of host susceptibility in the pathogenesis of RF (see Epidemiology section), and studies suggest that the abnormal immune response to GAS infection is genetically controlled (58,95,96). The major contributors to the autoimmune reaction in RF/RHD are the major histocompatibility complex HLA class 2 alleles (DR, DQ) located on human chromosome 6. Several HLA class II alleles have been associated with a greater likelihood of development of RF/RHD in different countries. Expressed on the surface of cells that present antigens, these HLA molecules are thought to participate in the development of both the humoral and cell-mediated responses to certain streptococcal antigens that ultimately result in immune-mediated inflammation resulting in the various manifestations of RF (97,98,99,100,101,102).
Several studies have shown a difference in the expression of a specific B-cell alloantigen detected by monoclonal antibody (D8/17) between patients with RF/RHD and control patients. In most of these studies, D8/17 is expressed in >85% of RF patients,
but <15% of controls. Furthermore, D8/17 expression has also been found to be higher in first-degree relatives of RF patients than in controls. A few studies have failed to confirm this association, possibly related to population differences. At present, the role of B-cell expression of this alloantigen in the immune response to GAS remains unclear, and there is no correlation between expression and clinical outcome (103,104,105,106).
but <15% of controls. Furthermore, D8/17 expression has also been found to be higher in first-degree relatives of RF patients than in controls. A few studies have failed to confirm this association, possibly related to population differences. At present, the role of B-cell expression of this alloantigen in the immune response to GAS remains unclear, and there is no correlation between expression and clinical outcome (103,104,105,106).
The genetics of other components of the immune response have also been evaluated. There is increasing evidence for the role of genetic polymorphisms (tumor necrosis factor alpha [TNF-α], angiotensin-converting enzyme, interleukins, others) in susceptibility to RF and the development of RHD (96,107,108,109,110,111,112,113).
Immunopathogenesis
Although our understanding of the pathogenesis of RF and RHD is incomplete, the importance of the host immune response to a preceding GAS infection is clear. One line of thinking, which includes the long-held belief in the concept of molecular mimicry, proposes the following: (1) GAS pharyngitis in a genetically susceptible host leads to breakdown products and streptococcal antigens that are cross-reactive with heart proteins (molecular mimicry) (114,115), (2) the immune response that occurs in response to the infection leads to cross-reactive antibodies and cytokine production (116,117), (3) the antibodies bind to the endothelial valve surface resulting in injury, cellular infiltration of inflammatory cells, and upregulation of vascular adhesion molecule 1 (VCAM-1), which aids in the recruitment and infiltration of T cells and macrophages, leading to further inflammation and damage (118), (4) endothelial injury exposes subendothelial structures and proteins including vimentin (found in cardiac fibroblasts) and laminin (extracellular matrix protein present in the basement membrane of valves and around endothelium), (5) inflammation leads to neovascularization, allowing further recruitment of T cells leading to granulomatous inflammation and many of the changes seen with chronic RHD, (6) cellular infiltration contributes to formation of Aschoff bodies, (7) activated B-lymphocytes and macrophages from Aschoff bodies express large amounts of HLA class II molecules on their surfaces and may play an important role in antigen presentation to T cells that have been recognized as important effectors of chronic rheumatic valvular disease (101), (8) infiltrating T cells are cross-reactive with streptococcal M protein and cardiac myosin and laminin (molecular mimicry); CD4+ T cells have been recognized as major effectors of this process leading to chronic RHD (119,120,121,122), and (9) inflammatory cytokines (increased amounts of TNF-α and interferon gamma (IFN-γ) along with decreased amounts of interleukin-4 (IL-4) appear to be important in persistence and progression of rheumatic valvular lesions in a susceptible host, (10) through a process called epitope spreading, T cells may respond to other cardiac α-helical proteins, including tropomyosin and vimentin (101,102,123,124,125) (Fig. 59.3).
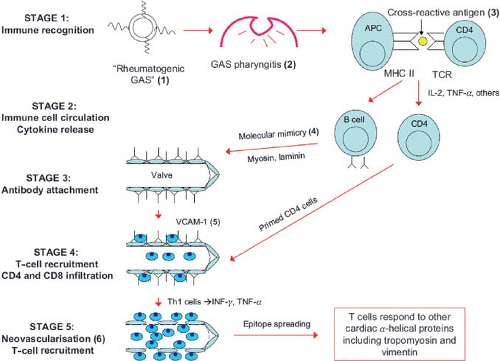 Figure 59.3 Immunopathogenesis or rheumatic fever and rheumatic heart disease. (1) and (2) Rheumatogenic group A streptococcal (GAS) pharyngitis. (3) Cross-reactive antigen on GAS. (4) Molecular mimicry between GAS antigens and human host tissue is believed to be the basis of pathogen–host cross-reactivity, best documented with α-helical cardiac proteins such as myosin, laminin, and vimentin. (5) Vascular cell adhesion molecule 1 (VCAM-1) is upregulated at the valve and aids in recruitment and infiltration of T cells. (6) Inflammation leads to neovascularization, which allows further recruitment of T cells, leading to granulomatous inflammation and the establishment of chronic rheumatic heart disease. (Adapted from Steer AC and Carapetis JR. Acute rheumatic fever and rheumatic heart disease in indigenous populations. Pediatr Clin N Am. 2009;56:1401–1419, with permission.) |
However, other investigators have recently proposed a different pathogenetic theory which does not involve molecular mimicry: (1) surface components of rheumatogenic strep form a complex with human collagen type IV in subendothelial basement membranes, (2) this complex initiates an autoantibody response to the collagen, resulting in an autoimmune response and collagen/endothelial cell inflammation and activation in patients with RF, (3) endothelial injury exposes subendothelial structures and proteins, leading to neovascularization, further recruitment of T cells, granulomatous inflammation, activated B cells and macrophages, changes in cytokines, and in some cases ongoing inflammation leading to scarring of rheumatic valvular tissue (as in the first proposed model of pathogenesis in the preceding paragraph) (126).
Natural History
The prognosis and natural history of rheumatic carditis and RHD are strongly influenced by both the severity of the initial carditis and RF recurrences (127,128,129,130,131,132). Mild carditis without recurrences is much more likely to show resolution than severe initial carditis and/or cases with recurrent episodes of RF. Only 30% to 40% of patients with acute mitral regurgitation have a persistent murmur at follow-up, with most of the clinical improvement occurring in the first 6 months after the acute illness. Patients with more severe carditis (heart failure and/or cardiomegaly) are more likely to have persistent RHD, and aortic regurgitation is less likely to decrease in severity or disappear than mitral regurgitation (133,134,135). The proportion of patients with RF who develop chronic RHD has decreased from 60–90% during the pre-penicillin era to 35–65% (127,128,129,136,137). Age and gender also influence prognosis, as acute rheumatic cardiac involvement resolves more frequently in boys (128,129), and children presenting with RF before age 5 years have more severe cardiac involvement and more commonly have persistent chronic RHD (44,45).
Pathology
The pathologic changes that occur with RF are characterized by inflammation of connective tissue in the heart, joints, and subcutaneous tissues. The pathologic changes in rheumatic carditis are primarily perivascular and interstitial, without evidence of myocyte necrosis. Two phases have been described. The “exudative” phase occurs in the first 2 to 3 weeks after disease onset and is characterized by interstitial edema, cellular infiltration (T cells, B cells, macrophages), fragmentation of collagen, and scattered deposition of fibrinoid (eosinophilic granular material). During the second “proliferative” or “granulomatous” phase, which lasts for months to years (138), the Aschoff nodule, considered pathognomonic for, and the morphologic hallmark of RHD, may be found in the endocardium, subendocardium, or myocardial interstitium (139). The Aschoff nodule is a perivascular aggregation characterized by a central area of fibrinoid change (altered collagen) surrounded by or infiltrated by large multinucleated (“owl eye”) cells. These Aschoff bodies, which are not seen in the hearts of patients dying within the first week after onset of RF, may be seen years after the initial RF illness and do not correlate with disease activity (138,140). Recent studies suggest that cells in Aschoff bodies located underneath activated valvular endothelium play an important role in antigen presentation to infiltrating T cells, which have been recognized as critical in the evolution of chronic RHD (101) (see Immunopathogenesis section).
Pericarditis
Grossly, the pericardial surface may have a white, fibrinous, stringy to shaggy exudate; all cases show lymphocytic and mononuclear infiltration of the pericardium. Aschoff nodules may be present in the pericardium. Pericarditis heals with no significant adhesions, and constrictive carditis rarely occurs.
Myocarditis
The ventricles and atria are often enlarged in RHD. Histologically, the myocardium may be edematous and show nonspecific inflammation. However, different from other forms of myocarditis, there is usually no evidence of cell damage. A variable degree of interstitial fibrinoid degeneration with inflammatory foci consisting of lymphocytes, macrophages, and other inflammatory cells has been reported as a common finding (141). Aschoff bodies may also be seen in locations of the myocardial interstitium. Despite frequent first-degree atrioventricular (AV) block on the ECG, the conduction system shows few pathologic changes (142,143).
Endocarditis
Endocardial inflammatory changes are responsible for valvulitis and are therefore the most clinically significant. Small, 1 to 2 mm, friable, fibrinous, verrucous vegetations may occur on the atrial surface of the mitral valve or on the ventricular side of the aortic valve at sites of valve closure (144). Associated underlying inflammation consists of histiocytes and lymphocytes.
The mitral valve leaflets may be edematous and vascularized. With time, granulation tissue may occur, with thickening and eventually fibrosis of the valve. Similarly, chordal inflammation may be followed by granulation tissue, fibrosis, and eventually chordal fusion. These changes may result in the mitral stenosis and regurgitation seen with chronic RHD.
Macroscopically, acute rheumatic mitral valvulitis results in elongation (or even rupture) of the chordae to the anterior mitral valve leaflet and annular dilation, resulting in altered leaflet coaptation, the potential for prolapse of the anterior leaflet, and mitral regurgitation (145,146). This altered coaptation most commonly results in a posterolaterally directed jet of MR, directed toward an area of fibrotic thickening of the posterior left atrial wall called “MacCallum’s patch.” Aortic valve prolapse has been proposed as one of the mechanisms contributing to acute rheumatic aortic regurgitation (147).
Vasculitis
Generalized vasculitis, in particular involving the coronary arteries and the aorta, has been described (148). It resembles changes of hypersensitivity angiitis, but rarely results in tissue damage or clinical manifestations.
Clinical Manifestations
Diagnosis and Evaluation
Since there is no pathognomonic test, the diagnosis of an initial episode of RF is made using the modified Jones or other diagnostic criteria. First proposed by T. Duckett Jones in 1944 (149), these criteria have undergone four revisions or modifications, the last in 1992 (150,151,152,153,154). Revisions and modifications have increased the specificity but decreased the sensitivity of the criteria to avoid overdiagnosis. Although this makes sense in developed countries where RF is now relatively uncommon, strict adherence to the Updated Jones Criteria in settings where RF continues to be common may result in underdiagnosis (155,156,157). The latest Updated Jones Criteria were published in 1992 and are intended to be used to establish the initial attack of
acute RF (Table 59.1) (154). The diagnosis of RF requires two major, or one major and two minor Jones criteria along with evidence of a preceding streptococcal infection. The major criteria are polyarthritis, carditis, chorea, a characteristic rash called erythema marginatum, and subcutaneous nodules. The frequency of major manifestations from several published series of RF cases is shown in Table 59.2. The minor criteria are fever, arthralgia, elevated acute phase reactants, and a prolonged PR interval on the electrocardiogram. Table 59.3 lists suggested testing for patients with suspected RF. Evidence of a preceding GAS infection is discussed below in the section on Laboratory Testing. The latest Update allows the diagnosis of RF to be made without fulfilling the above criteria in three circumstances: (1) patients who present with isolated chorea, (2) patients who present with indolent or insidious onset carditis (detected months to years after the acute illness), or (3) patients with a prior history of RF/RHD (154).
acute RF (Table 59.1) (154). The diagnosis of RF requires two major, or one major and two minor Jones criteria along with evidence of a preceding streptococcal infection. The major criteria are polyarthritis, carditis, chorea, a characteristic rash called erythema marginatum, and subcutaneous nodules. The frequency of major manifestations from several published series of RF cases is shown in Table 59.2. The minor criteria are fever, arthralgia, elevated acute phase reactants, and a prolonged PR interval on the electrocardiogram. Table 59.3 lists suggested testing for patients with suspected RF. Evidence of a preceding GAS infection is discussed below in the section on Laboratory Testing. The latest Update allows the diagnosis of RF to be made without fulfilling the above criteria in three circumstances: (1) patients who present with isolated chorea, (2) patients who present with indolent or insidious onset carditis (detected months to years after the acute illness), or (3) patients with a prior history of RF/RHD (154).
TABLE 59.1 Diagnostic Criteria for Rheumatic Fever | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Modifications of the Jones diagnostic criteria have been published in parts of the world where the incidence of RF and prevalence of RHD remain high, including Australia, New Zealand, and India (169,170,171). The Guidelines from India are similar to the 1992 Updated Jones Criteria. In Australia, the guidelines define different criteria for diagnosis in high-risk groups (see Table 59.1): (1) accepting subclinical (echocardiographically detected) findings as carditis, (2) accepting monoarthritis or polyarthralgia in addition to polyarthritis as a major criterion, and (3) accepting monoarthralgia as a minor criterion (171). In New Zealand, subclinical carditis is accepted as a major criterion (169). A recent analysis reported a 16% increase in cases of RF using the New Zealand Guidelines rather than the Updated 1992 Jones Criteria (172). The intent of these modifications is to improve the sensitivity of the diagnostic criteria in populations where RF and RHD are common and there is greater concern for underdiagnosis with its associated consequences. These modifications are presented in Table 59.1.
While the Jones and other diagnostic criteria may be used to diagnose a recurrent episode of RF in patients without RHD, diagnosing a recurrence may be difficult in the patient with existing RHD. In such cases, it is often difficult to differentiate acute carditis from progression and evolution of existing RHD. Such a distinction is important for at
least two reasons: (1) cardiac changes related to an acute recurrence are likely to evolve over a shorter time period than chronic RHD, (2) some believe that steroids may be beneficial and even lifesaving in patients with severe, active carditis. On the other hand, steroids are of no therapeutic value in patients with chronic rheumatic valvular disease, and might delay more appropriate treatment. The most recent Update of the Jones criteria states that “a presumptive diagnosis of rheumatic recurrence may be made when a single major or several minor manifestations are present in a patient with a reliable history of RF or RHD, provided there is supporting evidence of a recent group A streptococcal infection” (154). The Australia criteria for diagnosing a recurrence are 2 Major, or 1 Major + 1 Minor, or 3 Minor criteria + evidence of a preceding strep infection (171). In New Zealand, the criteria for diagnosing a recurrence are 2 Major, or 1 Major + 2 Minor, or several Minor + evidence of a preceding strep infection (169) (see Table 59.1).
least two reasons: (1) cardiac changes related to an acute recurrence are likely to evolve over a shorter time period than chronic RHD, (2) some believe that steroids may be beneficial and even lifesaving in patients with severe, active carditis. On the other hand, steroids are of no therapeutic value in patients with chronic rheumatic valvular disease, and might delay more appropriate treatment. The most recent Update of the Jones criteria states that “a presumptive diagnosis of rheumatic recurrence may be made when a single major or several minor manifestations are present in a patient with a reliable history of RF or RHD, provided there is supporting evidence of a recent group A streptococcal infection” (154). The Australia criteria for diagnosing a recurrence are 2 Major, or 1 Major + 1 Minor, or 3 Minor criteria + evidence of a preceding strep infection (171). In New Zealand, the criteria for diagnosing a recurrence are 2 Major, or 1 Major + 2 Minor, or several Minor + evidence of a preceding strep infection (169) (see Table 59.1).
TABLE 59.2 Major Manifestations of Rheumatic Fever | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 59.3 Testing for Patients with Suspected Rheumatic Fever | |
|---|---|
|
Arthritis
The latency period between GAS infection and most manifestations of RF ranges from 10 days to 5 weeks (latency between GAS infection and chorea is 1 to 6 months) (94). Of the major Jones criteria, migratory polyarthritis is most common, affecting 40% to 70% of cases (Table 59.2). The arthritis of RF classically migrates from large joint to large joint, and most commonly affects the knees, ankles, elbows, and wrists. Importantly, the presentation and evolution of the joint manifestations may be affected by administration of anti-inflammatory medications (aspirin or other nonsteroidal anti-inflammatory agents). It is noteworthy that in some parts of the world, monoarticular arthritis is a common mode of presentation (155,173). The joints affected with RF are red, swollen, and extremely tender, often with pain out of proportion to the objective findings. In some cases, the joints may be involved sequentially and simultaneously rather than in a migratory pattern, with a new joint becoming involved while a different joint is at a different phase of inflammation and resolution. Even untreated, the arthritis of RF usually resolves within 3 to 4 weeks and is not associated with residual abnormalities. Although carditis and arthritis commonly occur together, the severity of the joint and heart involvement tend to be inversely related (129). The reasons for this inverse relationship are unclear; some have speculated that joint involvement leads to earlier medical attention and initiation of anti-inflammatory treatment, thus preventing more severe cardiac involvement. Because of the different latency periods between the preceding streptococcal pharyngitis and the onset of symptoms, polyarthritis and chorea uncommonly occur simultaneously (174). The arthritis of RF typically responds to aspirin within 48 to 72 hours. In fact, lack of clinical response and improvement within 2 to 3 days should prompt consideration of alternative diagnoses (154,175). It is worth noting that a small subset of patients relapses once or twice after a 6-week course of antirheumatic therapy (176,177). Unfortunately, although arthritis is the most common major manifestation of RF, it is also the least specific, and is therefore the most common feature associated with misdiagnosis. The Jones criteria often fail to exclude other causes of febrile polyarthritis (48), and an alternative diagnosis may be made only as more chronic findings develop (i.e., autoimmune or collagen vascular disease). The differential diagnosis for polyarthritis and fever is presented in Table 59.4 (178).
TABLE 59.4 Causes of Polyarthritis and Fever | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Some patients develop arthritis after a streptococcal pharyngitis that differs from that typically associated with acute RF. This entity, termed poststreptococcal reactive arthritis, typically occurs after a shorter latent period (7 to 10 days), tends to be nonmigratory and more persistent, in some cases involves small joints or the axial skeleton, and does not respond as dramatically to anti-inflammatory medications as does the typical arthritis of RF (179,180,181). The important implication of diagnosing poststreptococcal reactive arthritis as opposed to RF is the risk for subsequent valvular heart disease and need for antistreptococcal prophylaxis. Of particular importance is the fact that some patients thought to have poststreptococcal reactive arthritis have shown evidence of cardiac involvement (179,180,182,183). Conversely, a recent study showed no increased risk of valvular heart disease in a series of adults with poststreptococcal reactive arthritis (184). Given the uncertainty with respect to the risk of valvular heart disease for children with poststreptococcal reactive arthritis, some experts recommend that such patients undergo echocardiographic evaluation, receive secondary prophylaxis for up to a year after onset, and possibly undergo a follow-up echocardiogram after a year (185), but this is clearly an area of debate requiring further study. If valvular heart disease is detected, such patients should be considered as having had RF and should be treated accordingly (including secondary prophylaxis) (180,182,184,186,187,188).
Chorea
First described in the late 17th century, the association of chorea and rheumatism was not recognized until the 19th century. It is now known that the clinical manifestations of Sydenham chorea occur due to neuropathologic changes and inflammation in the basal ganglia, cerebral cortex, and the cerebellum (48,189).
Sydenham chorea occurs in approximately 10% to 30% of cases of RF (see Table 59.2). The gender distribution is equal in younger children, but after the age of 10 years, females are more often affected, and chorea is uncommon in postpubertal males (48). Involuntary, purposeless movements, muscular incoordination and/or weakness, and emotional lability characterize Sydenham chorea (48,49,190,191,192). Movements are abrupt and erratic, commonly affecting muscles of the face and extremities. Findings may include “fidgetiness,” facial grimaces, tongue movements described as resembling a “bag of worms,” halting and explosive speech, pronation of the hands when arms are extended above the head (“pronator sign”), irregular contractions of the hands when asked to squeeze an object (“milkmaid’s grip”), hyperextension of the fingers when hands are extended forward with eyes closed (“spooning”), and clumsiness. Patients often come to attention based on deterioration in school performance, and neurobehavioral symptoms seen along with the chorea include irritability, poor attention span, lack of cooperation, and obsessive-compulsive symptoms are not uncommon. Sensory deficits do not occur. The neurologic manifestations are usually bilateral, but may be unilateral (hemichorea). The neurologic symptoms tend to decrease with rest and sedation and increase with effort or excitement. The duration of chorea is variable, ranging from 1–2 weeks to 2–3 years. The median duration is about 15 weeks, and 75% show resolution of symptoms by 6 months (5,169,193). Recurrent episodes of chorea are not uncommon (194). The differential diagnosis of chorea is presented in Table 59.5.
The latency period between the streptococcal pharyngitis and the onset of chorea is longer than for arthritis or carditis, ranging from 1 to 6 months (48,195). As previously stated, because of this longer latency period, arthritis and chorea rarely occur simultaneously. Also related to the longer latency period for patients presenting with chorea, acute phase reactants are often normal and antistreptococcal antibodies may not be elevated. When carditis and chorea are found in the same patient, it is often the chorea that prompts medical attention at which time rheumatic cardiac involvement is detected. Although the combination of chorea and carditis is common, occurring in 47% of patients with RF in a recent series (70), the cardiac involvement tends to be relatively mild and heart failure is uncommon (50). However, rheumatic cardiac involvement in these patients may evolve and progress over time, with some patients presenting with chronic RHD years after the RF illness. Even patients with “pure” chorea are at risk, however, as 20% to 44% of such patients go on to develop chronic RHD (127,174,190,196).
TABLE 59.5 Differential Diagnosis of Chorea | |
|---|---|
|
Neuroimaging (MRI or CT) abnormalities have been reported in some patients with Sydenham chorea (192,197). However, reported findings have been variable, and many patients have had normal imaging studies. Thus, neuroimaging should be reserved for atypical cases to exclude other causes of chorea. Similarly, although abnormalities have been described (198), EEG testing should be reserved for atypical cases.
In 1998, Swedo and colleagues proposed that, in some patients, childhood obsessive-compulsive symptoms and tics occur as a result of an autoimmune response to a preceding streptococcal infection, terming the entity PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections) (199,200). Because the proposed mechanism of autoimmunity related to cross-reactivity between streptococcal antigens and brain tissue is similar to the mechanism invoked for Sydenham chorea, it has been suggested that secondary prophylaxis might prevent recurrent neurologic symptoms. (201,202). In some patients, it can be difficult to differentiate choreiform movements from tics (190,203). Differentiating PANDAS from Sydenham chorea is important since the treatments and prognoses are different, in particular related to the risk of chronic RHD. Patients with PANDAS may respond to plasmapheresis or intravenous gamma globulin (204). Other manifestations of RF (including carditis and RHD) have not been reported in patients with PANDAS (200). At present, there continues to be considerable debate regarding the association of streptococcal infection and neuropsychiatric disorders (PANDAS), and some consider the entity as a yet-unproven hypothesis (187,205,206).
TABLE 59.6 Differential Diagnosis of Rheumatic Carditis | |
|---|---|
|
Carditis
The manifestation of RF associated with long-term morbidity and mortality is carditis, which occurs in 30% to 70% of cases (see Table 59.2). Rheumatic carditis remains the most common cause of acquired heart disease in children and young adolescents in developing countries. Despite traditionally being described as a pancarditis, the dominant and most important abnormality with acute rheumatic cardiac involvement is the valvulitis, specifically mitral and/or aortic regurgitation. The clinical presentation may be quite variable, ranging from the asymptomatic patient with a characteristic heart murmur to the critically ill patient presenting in heart failure. Severe carditis and heart failure occur in 13% to 64% of RF cases and represent 15% to 50% of patients with carditis (see Table 59.2) (70,127,129,155,158,159,160,161,162,163,164,165,166). Some of the variability is likely related to the fact that some patients present only after more than one episode of RF and carditis, a scenario much more likely to be associated with significant valvular disease and heart failure. Approximately 80% of patients who develop carditis do so within the first 2 weeks of the RF illness; if no cardiac involvement is detected in the first 2 weeks, the likelihood of subsequent cardiac involvement during the acute phase is low (207). The severity of carditis and valvular regurgitation often decreases as the inflammation subsides. If the cardiac involvement is mild, patients may show complete resolution of cardiac findings, but patients with moderate-to-severe carditis are more likely to experience persistent and/or evolving RHD (127,129). The differential diagnosis of carditis is presented in Table 59.6.
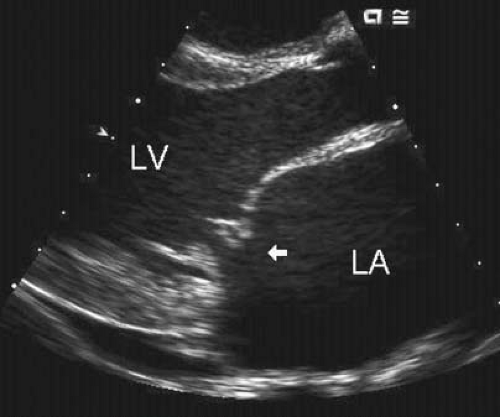
Mitral regurgitation, the dominant cardiac abnormality in patients with RF, occurs in approximately 95% of cases with acute rheumatic carditis. Both echocardiographic and surgical observations have demonstrated the mechanism of this mitral regurgitation to be a combination annular dilation and chordal elongation that results in abnormal coaptation, and in some cases, prolapse of the tip of the anterior mitral leaflet (Figs. 59.4 and 59.5) (208,209,210). Rarely, the mitral valve chordae rupture, resulting in a flail mitral leaflet and severe mitral regurgitation (190,211,212,213) (see Fig. 59.5).
Most patients with acute mild mitral regurgitation are asymptomatic. With acute moderate-to-severe mitral regurgitation, the left ventricular myocardium may be unable to handle the significant acute volume overload, leading to a rise in left heart filling pressures, pulmonary venous congestion, and pulmonary edema. Such patients usually present with features of left heart failure, including dyspnea, orthopnea, paroxysmal nocturnal dyspnea, cough, and even hemoptysis. Secondary pulmonary hypertension may develop, resulting in right ventricular dysfunction, tricuspid regurgitation, and right heart failure. Children younger than 5 years of age with RF and carditis may present more insidiously with fever, decreased appetite, lethargy, fatigue, and vague pains. Because of these subtle and nonspecific symptoms, the diagnosis may be delayed, and presentation with heart failure is more common than in older children (44,45). On physical examination, tachycardia is often one of the earliest signs of carditis. Significant mitral regurgitation may result in increased precordial activity, tachypnea, and increased work of breathing. A high-pitched, regurgitant, holosystolic murmur of mitral regurgitation is heard best at the apex, usually radiating into the left axilla. This murmur is best heard at end-expiration with the patient in the left lateral decubitus position. It is noteworthy that acute, severe mitral regurgitation may be present despite a fairly soft systolic murmur (214). Although mitral stenosis does not occur with the initial episode of acute RF and carditis, a low-pitched mid-diastolic apical murmur may be heard in the setting of significant mitral regurgitation due to increased diastolic flow across the mitral valve (Carey Coombs murmur) (215). Contrary to some reports (154), this murmur is never heard in isolation.
Aortic regurgitation occurs in approximately 20% to 25% of patients with acute rheumatic carditis, usually in combination with mitral regurgitation. Isolated aortic regurgitation occurs in approximately 5% of patients with acute rheumatic carditis (70,162). Leaflet prolapse has been reported to be one of the mechanisms of this acute valvular dysfunction (147,210). Patients with acute mild aortic regurgitation are usually asymptomatic. Moderate-to-severe
acute aortic regurgitation is less well tolerated, and may result in heart failure. The large regurgitant volume imposed on a left ventricle that has not had time to compensate for the significant volume load results in decreased forward stroke volume in conjunction with significant elevation of left heart filling pressures, leading to a combination of low cardiac output and pulmonary edema. Patients with acute severe aortic regurgitation are tachycardic and tachypneic. Unlike the clinical examination found with significant chronic aortic regurgitation, the pulse pressure is often narrow and the pulses are not increased or bounding. Precordial activity is often increased, but the apical impulse may not be significantly displaced. On auscultation, the decrescendo diastolic murmur is softer, lower pitched, and shorter than the murmur heard with chronic regurgitation. Thus, this murmur can be easily missed, especially with the tachycardia commonly present during the acute phase of the illness. A short systolic ejection murmur may be heard over the left ventricular outflow tract due to increased flow. A low-pitched mid-to-late diastolic rumbling murmur with presystolic accentuation may be heard at the apex, even with a nonstenotic mitral valve. If present, this “Austin Flint” murmur is softer and shorter in the setting of acute as compared to chronic severe aortic regurgitation. Acute rheumatic aortic regurgitation is less likely than mitral regurgitation to disappear with resolution of the acute inflammatory stage of the illness (127,129,133).
acute aortic regurgitation is less well tolerated, and may result in heart failure. The large regurgitant volume imposed on a left ventricle that has not had time to compensate for the significant volume load results in decreased forward stroke volume in conjunction with significant elevation of left heart filling pressures, leading to a combination of low cardiac output and pulmonary edema. Patients with acute severe aortic regurgitation are tachycardic and tachypneic. Unlike the clinical examination found with significant chronic aortic regurgitation, the pulse pressure is often narrow and the pulses are not increased or bounding. Precordial activity is often increased, but the apical impulse may not be significantly displaced. On auscultation, the decrescendo diastolic murmur is softer, lower pitched, and shorter than the murmur heard with chronic regurgitation. Thus, this murmur can be easily missed, especially with the tachycardia commonly present during the acute phase of the illness. A short systolic ejection murmur may be heard over the left ventricular outflow tract due to increased flow. A low-pitched mid-to-late diastolic rumbling murmur with presystolic accentuation may be heard at the apex, even with a nonstenotic mitral valve. If present, this “Austin Flint” murmur is softer and shorter in the setting of acute as compared to chronic severe aortic regurgitation. Acute rheumatic aortic regurgitation is less likely than mitral regurgitation to disappear with resolution of the acute inflammatory stage of the illness (127,129,133).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree