Bongani M. Mayosi
Rheumatic Fever
Rheumatic fever is the leading cause of acquired heart disease in children and young adults worldwide. Initiated by a group A beta-hemolytic streptococcal (GAS) pharyngeal infection and following a latent period of approximately 2 to 3 weeks, the illness is characterized by acute inflammation of the heart, joints, skin, subcutaneous tissue, and central nervous system. Pathologically, the inflammatory process causes damage to collagen fibrils and connective tissue ground substance (i.e., fibrinoid degeneration), and thus rheumatic fever is classified as a connective tissue or collagen-vascular disease.
It is the destructive effect on the heart valves that leads to the chronic sequelae of the disease (i.e., rheumatic heart disease), with serious hemodynamic disturbances causing cardiac failure, as well as other complications such as stoke and infective endocarditis. Referring to the fleeting arthritis and damaging carditis characteristic of rheumatic fever, the French physician Ernst-Charles Lasègue famously said in 1884, “Pathologists have long known that rheumatic fever licks at the joints, but bites at the heart.” Almost all cases of rheumatic fever and rheumatic heart disease and associated deaths are entirely preventable.
Epidemiology
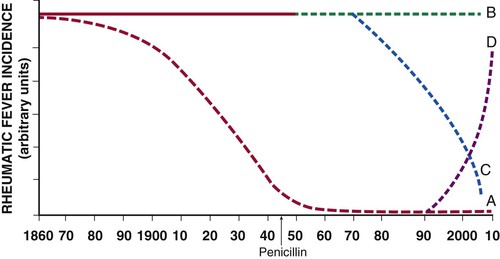
The burden of rheumatic fever and rheumatic heart disease has been characterized by at least four changing patterns over the past 150 years (Fig. 83-1). The first pattern represents the preantibiotic fall in the incidence of rheumatic fever that is typical of industrialized countries (curve A, Fig. 83-1). For example, in the United States the incidence per 100,000 was 100 at the start of 20th century, 45 to 65 between 1935 and 1960, and currently estimated to be less than 10 cases per 100,000.1 The decrease in the incidence of rheumatic fever preceded the introduction of antibiotics in the 1940s and is almost certainly due to improved socioeconomic standards, less overcrowded housing, and improved access to medical care.
The second pattern is characterized by a persistently high incidence of rheumatic fever in developing regions that do not have any comprehensive national programs of primary and secondary prevention (curve B, Fig. 83-1). The incidence of rheumatic fever in Maori children is as high as 59 per 100,000 per year, as compared with 1.1 per 100,000 in the non-Maori community of New Zealand.2 This hyperendemic pattern of rheumatic fever affects most of the population of the world who live in Africa, the Middle East, Asia, eastern Europe, South America, and indigenous communities of Australasia.3
Third, some developing countries, such as Cuba, Costa Rica, and the French islands of Martinique and Guadalupe, have experienced a falling incidence of rheumatic fever following the implementation of comprehensive public health programs for primary and secondary prevention of rheumatic fever (curve C, Fig. 83-1).4 By contrast, African countries that have not implemented public health programs for the prevention of rheumatic fever continue to experience a high incidence of rheumatic fever and rheumatic heart disease.5
Outbreaks of rheumatic fever have been reported in affluent communities of the United States and Italy.6 The epidemiologic transition in the former Soviet Union has been associated not only with an increase in mortality rates from atherosclerotic diseases and trauma in Russia but also with a sustained resurgence of rheumatic fever and rheumatic heart disease in central Asia.7 The incidence of rheumatic fever in central Asia fell to the same levels as those in Japan in the middle 1970s but rose sharply in the post-Soviet period to levels associated with developing countries (curve D, Fig. 83-1). In developing countries, Kyrgyzstan probably has the highest incidence of rheumatic fever and rheumatic heart disease—approximately 543 per 100,000 population per year—thus earning the central Asian republics the dubious distinction of being the rheumatic fever “hot spot” of the world. The resurgence of rheumatic fever in the formerly Soviet republics may reflect weakening of the primary health care system and the economic crisis of the post-Soviet period (see Tulchinsky and colleagues in Classic Reading List).
Pathogenesis
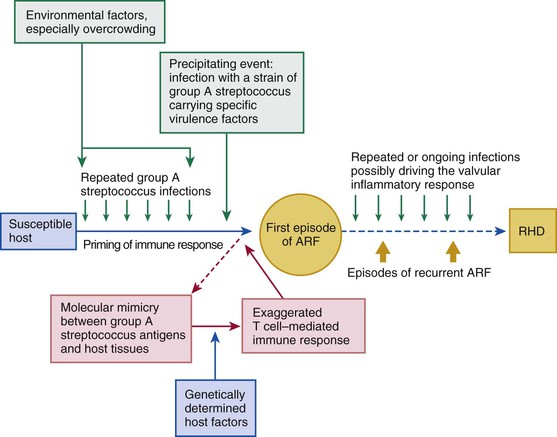
Rheumatic fever is a multifactorial disease that follows GAS pharyngitis (the agent) in a susceptible individual (the host) who lives under deprived social conditions (the environment). The theory of molecular mimicry holds that GAS pharyngitis triggers an autoimmune response to epitopes in the organism that cross-react with similar epitopes in the heart, brain, joints, and skin, and repeated episodes of rheumatic fever lead to rheumatic heart disease (Fig. 83-2).8
Clinical Features
The typical attack of rheumatic fever follows an episode of streptococcal pharyngitis after a latent period of 2 to 3 weeks. During the latent period there is no clinical or laboratory evidence of active inflammation. However, in as many as a third of patients in whom rheumatic fever develops, it does so after an asymptomatic GAS infection, and in outbreaks, up to 58% of patients have no symptoms of pharyngitis. This is one of the potential barriers to the effectiveness of primary prevention of rheumatic fever solely with antibiotic treatment of GAS pharyngitis and provides justification for the development of an anti-GAS vaccine as one of the strategies for control of rheumatic fever and other streptococcal diseases.
Rheumatic fever occurs most commonly in children between the ages of 4 and 9 years. In developing countries such as Saudi Arabia and India, juvenile mitral stenosis may occur at the age of 3 to 5 years.19 The prevalence of the various clinical features varies in different studies depending on whether the patients are studied prospectively or in retrospect. The illness usually begins with a high fever, but in some patients the fever may be low grade or absent. The most common of the major criteria is polyarthritis, which occurs in two thirds to three quarters of patients, followed by carditis and chorea.
Arthritis
Joint involvement is more common (almost 100%) and more severe in young adults than in teenagers (82%) and children (66%).20 The joint pain is typically described as migratory, which refers to the sequential involvement of joints, with inflammation resolving in one joint and then beginning in another. Sometimes the joint involvement may be additive rather than migratory, with simultaneous involvement of several joints. In untreated patients the number of joints involved may vary from 6 to 16.20
The affected joint may be inflamed for only a few days to a week before the inflammation subsides. In approximately two thirds of patients, the polyarthritis is severe for around a week and may last another 1 to 2 weeks in the remainder before it resolves completely. If joint swelling persists after 4 weeks, it is necessary to consider other conditions such as juvenile idiopathic arthritis or systemic lupus erythematosus.20
At the onset of the illness, joint involvement is asymmetric, with the lower limbs generally being affected initially before spreading to the upper limbs. Monarthritis has been reported in 17% to 25% of patients.21 Large joints such as the knees, ankles, elbows, and wrists are involved most frequently. The hip, shoulder, and small joints of the hands and feet are involved less frequently. Analysis of synovial fluid has shown the presence of sterile inflammatory fluid. There may be a reduction in complement components C1q, C3, and C4, thus suggesting their consumption by immune complexes. Radiographs may show features of a joint effusion, but no other abnormalities are noted.20
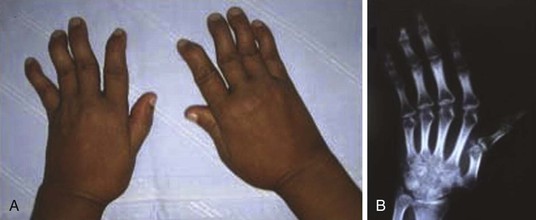
Jaccoud arthritis or arthropathy (or chronic post–rheumatic fever arthropathy) is a rare manifestation of rheumatic fever characterized by deformities of the fingers and toes (Fig. 83-4). The condition may occur after repeated attacks of rheumatic fever and results from recurrent inflammation of the fibrous articular capsule. There is ulnar deviation of the fingers, especially the fourth and fifth fingers, flexion of the metacarpophalangeal joints, and hyperextension of the proximal interphalangeal joints (i.e., swan neck deformity). The hand is usually painless without any signs of inflammation. The deformities are generally correctible but may become fixed in the later stages. No true erosions are seen on radiographs, and rheumatoid factor is usually negative. A similar form of arthropathy is seen in patients with systemic lupus erythematosus.20
The arthritis of rheumatic fever responds promptly to nonsteroidal anti-inflammatory drugs, and thus the classic finding of migratory polyarthritis may be infrequent at places in which self-medication with anti-inflammatories or their prescription without considering the diagnosis is common. The apparent fall in the incidence of rheumatic fever in some developing countries may be related to the indiscriminate use of nonsteroidal anti-inflammatory drugs without considering a diagnosis of rheumatic fever.22 The differential diagnosis of polyarticular arthritis in children and adolescents includes poststreptococcal reactive arthritis, other autoimmune diseases, septic arthritis, infective endocarditis, Lyme disease, lymphoma/leukemia, viral arthropathy, and sickle cell disease.
Poststreptococcal reactive arthritis is diagnosed in patients who have an arthritis that is not typical of rheumatic fever but who have evidence of recent streptococcal infection. This condition is said to develop after a shorter latent period than occurs with rheumatic fever, responds less well to anti-inflammatories, and may be associated with renal manifestations; evidence of carditis is not usually seen. The distinction between poststreptococcal reactive arthritis and rheumatic fever is unclear, and many would recommend that a diagnosis of poststreptococcal reactive arthritis not be made in populations in which rheumatic fever is common. Even if the diagnosis is considered, it is appropriate to offer a period of secondary penicillin prophylaxis as for episodes of acute rheumatic fever in such populations.23
Carditis
Carditis is the most serious manifestation of rheumatic fever in that it may lead to chronic rheumatic heart disease with its attendant complications of atrial fibrillation, stroke, heart failure, infective endocarditis, and death. In some cases the carditis may be asymptomatic and is detected during clinical examination of a patient with arthritis or chorea. The incidence of carditis during the initial attack of rheumatic fever varies from 40% to 91%, depending on the selection of patients and whether the diagnosis is made by clinical assessment alone or combined with echocardiography.23
The incidence of carditis complicating rheumatic fever varies with the age of the patient. It is reported in 90% to 92% of children younger than 3 years, in 50% of children 3 to 6 years of age, in 32% of teenagers aged 14 to 17 years, and in only 15% of adults with a first attack of rheumatic fever.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree