James C. Fang, Patrick T. O’Gara
The History and Physical Examination
An Evidence-Based Approach
Evaluation of the patient with known or suspected cardiovascular disease begins with a directed history and targeted physical examination, the scope of which depends on the clinical context of the patient encounter. Elective, ambulatory investigations allow comparatively more time for the development of a comprehensive assessment, whereas emergency department visits and urgent bedside consultations necessitate a more focused strategy. The elicitation of the history, with its emphasis on major cardiovascular symptoms and their change over time, demands a direct interaction, neither delegated nor inferred from information gleaned from a cursory chart review. The history also affords a unique opportunity to assess the patient’s personal attitudes, intelligence, comprehension, acceptance or denial, motivation, fear, and prejudices. Such insights allow a more informed understanding of the patient’s preferences and values regarding shared decision making. The interview also can reveal genetic or familial influences and the impact of other medical conditions on the manifesting illness. Although time constraints have limited the emphasis on careful history taking,1 the information gathered from the patient interview remains essential to inform the design of a resource-sensitive diagnostic and treatment plan.
Physical examination skills also have declined. Only a minority of internal medicine and family practice residents recognize classic cardiac findings in relevant diseases. Performance does not predictably improve with experience.2 Residency work hours and health care system efficiency standards have severely restricted the time and expertise devoted to the mentored cardiovascular examination. In turn, less attention to bedside skills has increased the use of noninvasive imaging. Educational efforts, including repetition, patient-centered teaching conferences, and visual display feedback of auscultatory and Doppler echocardiographic findings, can improve performance.3–5
The evidence base that justifies correlations between history and physical examination findings and cardiovascular disease severity and prognosis has been most rigorously established for heart failure, valvular heart disease, and coronary artery disease. For example, vital signs and detection of pulmonary congestion and mitral regurgitation (MR) contribute importantly to bedside risk assessment in patients with acute coronary syndromes (ACSs).6,7 The diagnosis of heart failure in ambulatory patients derives from attention to three basic elements of the history and six elements of the physical examination. The three important features of the history are dyspnea at one flight of stairs, orthopnea, and paroxysmal nocturnal dyspnea. The six elements of the physical examination that have undergone validation are a displaced apex beat, rales, an irregularly irregular pulse, a heart murmur suggestive of MR, a heart rate greater than 60 beats/min, and an elevated jugular venous pressure (JVP).8 Accurate auscultation provides important insight into many valvular and congenital heart lesions.9
This chapter reviews the fundamentals of the cardiovascular history and physical examination in light of the evidence base from correlative studies. See earlier editions of this book for further details.
The History
The major signs and symptoms associated with cardiac disease include chest discomfort, dyspnea, fatigue, edema, palpitations, and syncope. In most cases, careful attention to the specific characteristics of chest discomfort—quality, location, radiation, triggers, mode of onset, and duration—along with alleviating factors and associated symptoms can narrow the differential diagnosis (see also Chapter 50). Cough, hemoptysis, and cyanosis also can contribute in this regard. Claudication, limb pain, edema, and skin discoloration usually indicate a vascular disorder. The cardiovascular clinician also should be familiar with common manifestations of acute stroke and transient ischemic attack, such as sudden weakness, sensory loss, incoordination, and visual disturbance. Angina pectoris must be differentiated from the pain associated with pulmonary embolism, pericarditis, aortic dissection, esophageal reflux, or costochondritis.
Several aspects of the presenting symptom of chest pain increase or decrease the likelihood of ACS. For example, pain that is sharp (likelihood ratio [LR], 0.3; 95% CI, 0.2 to 0.5), pleuritic (LR, 0.2; 95% CI, 0.1 to 0.3), positional (LR, 0.3; 95% CI, 0.2 to 0.5), or reproducible with palpation (LR, 0.3; 95% CI, 0.2 to 0.4) usually is noncardiac, whereas discomfort that radiates to both arms or shoulders (LR, 4.1; 95% CI, 2.5 to 6.5) or is precipitated by exertion (LR, 2.4; 95% CI, 1.5 to 3.8) has a higher likelihood of reflecting an ACS.10 Less classic symptoms (i.e., anginal equivalents) such as indigestion, belching, and dyspnea also should command the clinician’s attention when other features of the presentation suggest ACS, even in the absence of chest discomfort. Women, elderly persons, and patients with diabetes more commonly present with a less typical clinical picture. Dyspnea may occur with exertion or in recumbency (orthopnea) or even on standing (platypnea). Paroxysmal nocturnal dyspnea of cardiac origin usually occurs 2 to 4 hours after onset of sleep; the dyspnea is of sufficient severity to compel the patient to sit upright or stand and then subsides gradually over several minutes. The patient’s partner should be questioned about any signs of sleep-disordered breathing, such as loud snoring or periods of apnea. Pulmonary embolism often is associated with dyspnea of sudden onset.
Patients may use a variety of terms to describe their awareness of the heartbeat (palpitations), such as “flutters,” “skips,” or “pounding.” The likelihood of a cardiac arrhythmia modestly increases with a known history of cardiac disease (LR, 2.03; 95% CI, 1.33 to 3.11) and decreases when symptoms resolve within 5 minutes (LR, 0.38; 95% CI, 0.22 to 0.63) or when associated with panic disorder (LR, 0.26; 95% CI, 0.07 to 1.01).11 A report of a regular, rapid-pounding sensation in the neck (LR, 177; 95% CI, 25 to 1251) or visible neck pulsations associated with palpitations (LR, 2.68; 95% CI, 1.25 to 5.78) increases the likelihood that atrioventricular nodal reentrant tachycardia (AVNRT) is the responsible arrhythmia. The absence of a regular, rapid-pounding sensation in the neck makes detecting AVNRT much less likely (LR, 0.07; 95% CI, 0.03 to 0.19).12 Cardiac syncope occurs suddenly, with rapid restoration of full consciousness thereafter. Patients with neurocardiogenic syncope may experience an early warning sign (nausea, yawning), appear ashen and diaphoretic, and revive more slowly, albeit without signs of seizure or a prolonged postictal state. The complete history consists of information pertaining to traditional cardiovascular risk factors, a general medical history, occupation, social habits, medications, drug allergies or intolerance, family history, and systems review.
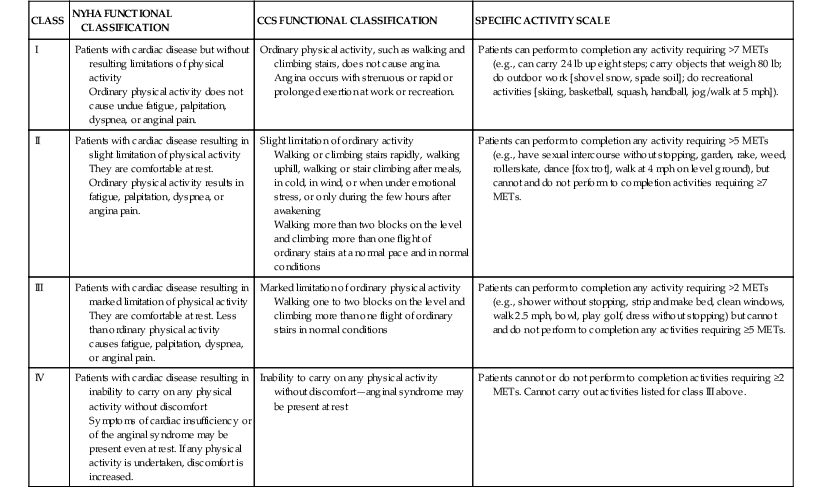
It is important to obtain a semiquantitative assessment of symptom severity and to document any change over time. The New York Heart Association (NYHA) and the Canadian Cardiovascular Society (CCS) functional classification systems are useful for both patient care and clinical research, despite their inherent limitations (Table 11-1).11,13
The General Physical Examination
The physical examination can help determine the cause of a given symptom, assess disease severity and progression, and evaluate the impact of specific therapies. It also can identify the presence of early-stage disease in patients without signs or symptoms.
General Appearance
The examination begins with an appreciation of the general appearance of the patient, including age, posture, demeanor, and general health status. Is the patient in pain, resting quietly, or visibly diaphoretic with a foreboding sense of doom? Does the patient choose to avoid certain positions to reduce or eliminate pain? The pain of acute pericarditis, for example, often diminishes with sitting up, leaning forward, or breathing shallowly. Pursing of the lips, a breathy quality to the voice, and an increased anteroposterior chest diameter would favor a pulmonary rather than a cardiovascular cause of dyspnea, although disorders in both etiologic categories may contribute in an individual patient. Pallor suggests anemia as a possible underlying disorder in patients with exercise intolerance or dyspnea, independent of cardiovascular disease. Cyanosis and jaundice also bear noting. Specific genetic cardiovascular disorders may be discernible from the patient’s appearance. Emaciation suggests chronic heart failure or another systemic disorder (e.g., malignancy, infection). The vital signs, including height, weight, temperature, pulse rate, blood pressure (in both arms), respiratory rate, and peripheral oxygen saturation, dictate the pace and scope of the evaluation and provide initial clues as to the presence of a cardiovascular disorder. The height and weight permit calculation of body mass index (BMI) and body surface area (BSA). Waist circumference (measured at the iliac crest) and waist-to-hip ratio (using the widest circumference around the buttocks) powerfully predict long-term cardiovascular risk.14,15 In patients with palpitations, a resting heart rate less than 60 beats/min may increase the likelihood of a clinically significant arrhythmia (LR, 3.00; 95% CI, 1.27 to 7.08).11 Observation of the respiratory pattern may reveal signs of disordered breathing (e.g., Cheyne-Stokes respirations, obstructive sleep apnea), a finding associated with reduced survival in patients with severe systolic heart failure.16 Mental status should be assessed.
Skin
Central cyanosis is present with significant right-to-left shunting at the level of the heart or lungs. It also is a feature of hereditary methemoglobinemia. Peripheral cyanosis or acrocyanosis of the fingers, toes, nose, and ears is characteristic of the reduced blood flow that accompanies small-vessel constriction seen in severe heart failure, shock, or peripheral vascular disease. Differential cyanosis affecting the lower but not the upper extremities occurs with a patent ductus arteriosus (PDA) and pulmonary artery hypertension with right-to-left shunting at the great vessel level. Hereditary telangiectases on the lips, tongue, and mucous membranes (a finding in Osler-Weber-Rendu syndrome) resemble spider nevi; when present in the lungs, they can cause right-to-left shunting and central cyanosis. Telangiectasias also are seen in patients with scleroderma with or without pulmonary hypertension. Tanned or bronze discoloration of the skin in unexposed areas can suggest iron overload and hemochromatosis. With jaundice, often first appreciated in the sclerae, the differential diagnosis is broad in scope. Ecchymoses often occur with either anticoagulant and/or antiplatelet use, whereas petechiae characterize thrombocytopenia, and purpuric skin lesions can be seen with infective endocarditis and other causes of leukocytoclastic vasculitis. Various lipid disorders can manifest with xanthomas, located subcutaneously, along tendon sheaths, or over the extensor surfaces of the extremities. Xanthomas within the palmar creases are specific for type III hyperlipoproteinemia. The leathery, cobblestone, “plucked chicken” appearance of the skin in the axillae and skinfolds of a young person is characteristic of pseudoxanthoma elasticum, a disease with multiple cardiovascular manifestations, including premature atherosclerosis.17 Extensive lentiginoses (freckle-like brown macules and café-au-lait spots over the trunk and neck) may be part of developmental delay–associated cardiovascular syndromes (LEOPARD, LAMB, and Carney) with multiple atrial myxomas, atrial septal defect (ASD), hypertrophic cardiomyopathy, and valvular stenoses. In a patient with heart failure or syncope, cardiovascular sarcoid should be suspected in the presence of lupus pernio, erythema nodosum, or granuloma annulare. Certain vascular disorders such as erythromelalgia or lymphangitis also may be readily apparent from examination of the skin.
Head and Neck
All patients should undergo assessment of the state of dentition, both as a source of infection and as an index of general health and hygiene. A high-arched palate is a feature of Marfan and other connective tissue disease syndromes. A large protruding tongue with parotid enlargement may suggest amyloidosis. A bifid uvula has been described in patients with Loeys-Dietz syndrome. Orange tonsils are characteristic of Tangier disease. Ptosis and ophthalmoplegia suggest muscular dystrophies, and congenital heart disease often is accompanied by hypertelorism, low-set ears, micrognathia, and a webbed neck, as with Noonan, Turner, and Down syndromes. Proptosis, lid lag, and stare point to Graves hyperthyroidism. Blue sclerae, mitral or aortic regurgitation (AR), and a history of recurrent nontraumatic skeletal fractures are observed in patients with osteogenesis imperfecta.
Attention to the extraocular movements and the size and symmetry of the pupils may reveal a neurologic disorder. The oft-omitted funduscopic examination can aid in the evaluation of patients with hypertension, atherosclerosis, diabetes, endocarditis, neurologic signs or symptoms, or known carotid or aortic arch disease. Lacrimal gland hyperplasia is sometimes a feature of sarcoidosis. The “mitral facies” of rheumatic mitral stenosis (pink-purplish patches with telangiectasias over the malar eminences) also can accompany other disorders associated with pulmonary hypertension and reduced cardiac output. Relapsing polychondritis is suggested by inflammation of the pinnae and nasal cartilage in association with a saddle-nose deformity. Palpation of the thyroid gland assesses its size, symmetry, and consistency. In some patients, earlier treatment with mantle irradiation for lymphoma can lead to a “dropped head myopathy,” characterized by loss of the anterior cervical strap muscles and permanent forward flexion.
Extremities
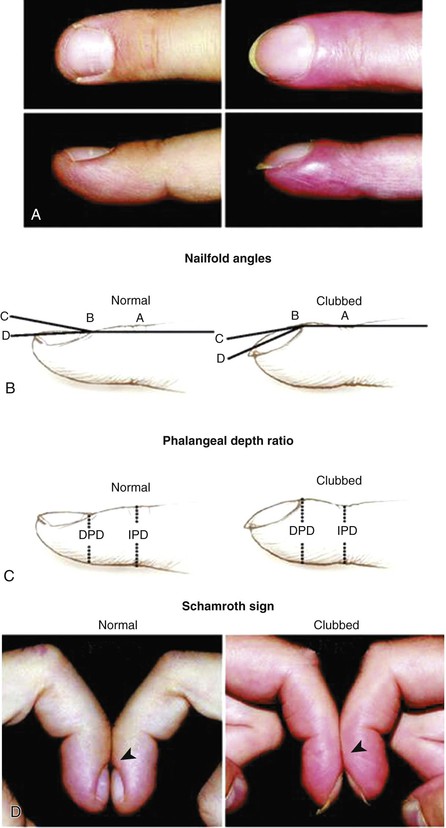
The temperature of the extremities and the presence of clubbing, arachnodactyly, and nail changes can be quickly ascertained. Clubbing implies the presence of central shunting (Fig. 11-1). The unopposable “fingerized” thumb occurs in Holt-Oram syndrome. Arachnodactyly characterizes the Marfan syndrome. Janeway lesions (nontender, slightly raised areas of hemorrhage on the palms and soles), Osler’s nodes (tender, raised nodules on the pads of the fingers or toes), and splinter hemorrhages (linear petechiae in the mid-nailbed) may be signs of infective endocarditis.
Lower extremity or presacral edema with elevated JVP occurs in many volume-overloaded states, including heart failure. With a normal JVP, additional signs of venous disease, such as extensive varicosities, medial ulcers, or brownish pigmentation from hemosiderin deposition, suggest chronic venous insufficiency. Edema also can occur with dihydropyridine calcium channel blocker therapy. Anasarca seldom occurs in heart failure, unless the condition is long standing, untreated, and accompanied by hypoalbuminemia. Asymmetric swelling can reflect local or unilateral venous thrombosis, the sequelae of previous vein graft harvesting, or lymphatic obstruction. Homan’s sign (calf pain elicited by forceful dorsiflexion of the foot) is neither specific nor sensitive for deep vein thrombosis. Muscular atrophy and the absence of hair in an extremity should suggest chronic arterial insufficiency or a neuromuscular disorder. Redistribution of fat from the extremities to central/abdominal stores (lipodystrophy) in some patients with HIV infection may relate to antiretroviral treatment, and is associated with insulin resistance and several features of the metabolic syndrome.
Chest and Abdomen
Cutaneous venous collaterals over the anterior chest suggest chronic obstruction of the superior vena cava (SVC) or subclavian vein, especially in the presence of indwelling catheters or leads. Asymmetric breast enlargement unilateral to an implanted device also may be present. Thoracic cage abnormalities, such as pectus carinatum (pigeon chest) or pectus excavatum (funnel chest), may accompany connective tissue disorders; the barrel chest of emphysema or advanced kyphoscoliosis may be associated with cor pulmonale. The severe kyphosis of ankylosing spondylitis should prompt careful auscultation for AR. The “straight back syndrome” (loss of normal kyphosis of the thoracic spine) can accompany mitral valve prolapse (MVP). A thrill may be present over well-developed intercostal artery collaterals in patients with aortic coarctation.
Patients with emphysema may exhibit prominence of the cardiac impulse in the epigastrium. The liver often is enlarged and tender in heart failure; systolic hepatic pulsations signify severe tricuspid regurgitation (TR). Patients with infective endocarditis of long duration may have splenomegaly. Ascites can develop with advanced and chronic right heart failure or constrictive pericarditis. The abdominal aorta normally may be palpated between the epigastrium and the umbilicus in thin patients and in children. The sensitivity of palpation for the detection of abdominal aortic aneurysm (AAA) disease increases as a function of aneurysm diameter and varies inversely with body size. Arterial bruits in the abdomen should be sought.
The Cardiovascular Examination
Jugular Venous Pressure and Waveform
The JVP aids in the estimation of volume status. The external (EJV) or internal (IJV) jugular vein may be used, although the IJV is preferred because the EJV is valved and not directly in line with the SVC and right atrium. The EJV is easier to visualize when distended, and its appearance can help to discriminate between low and high central venous pressure (CVP). An elevated left EJV pressure may also signify a persistent left-sided SVC or compression of the innominate vein from an intrathoracic structure. If an elevated CVP is suspected but venous pulsations cannot be appreciated, the patient should be asked to sit with the feet dangling. With subsequent pooling of blood in the lower extremities, venous pulsations may be evident. SVC syndrome should be suspected if the venous pressure is elevated, pulsations are still not discernible, and the skin of the head and neck appears dusky or cyanotic. When hypovolemia is suspected as a cause of hypotension, the patient may need to be lowered to a supine position to gauge the waveform in the right supraclavicular fossa.
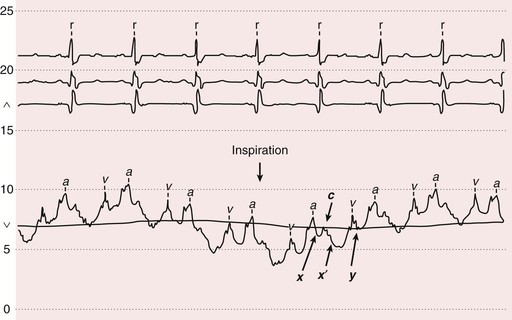
The venous waveform can sometimes be difficult to distinguish from the carotid artery pulse. The venous waveform has several characteristic features (Fig. 11-2; Table 11-2) and its individual components can usually be identified. The a and v waves, and x and y descents, are defined by their temporal relation to electrocardiographic events and heart sounds (S1 and S2, plus S3 and S4 as defined further on). The estimated height of the venous pressure indicates the CVP or right atrial pressure. Although observers vary widely in estimation of the CVP, knowledge that the pressure is elevated, and not its specific value, can inform diagnosis and management.
TABLE 11-2
Distinguishing Jugular Venous Pulse from Carotid Pulse
| FEATURE | INTERNAL JUGULAR VEIN PULSE | CAROTID ARTERY PULSE |
| Appearance of pulse | Undulating two troughs and two peaks for every cardiac cycle (biphasic) | Single brisk upstroke (monophasic) |
| Response to inspiration | Height of column falls and troughs become more prominent | No respiratory change to contour |
| Palpability | Generally not palpable (except in severe TR) | Palpable |
| Effect of pressure | Can be obliterated with gentle pressure at base of vein/clavicle | Cannot be obliterated |
TR = tricuspid regurgitation.
The venous pressure is measured as the vertical distance between the top of the venous pulsation and the sternal inflection point, where the manubrium meets the sternum (angle of Louis). A distance of greater than 3 cm is considered abnormal, but the distance between the angle of Louis and the mid–right atrium varies considerably, especially in obese patients. On chest computed tomography (CT) scans in 160 consecutive patients, this distance varied considerably in accordance with body position.18 In general, use of the sternal angle as a reference leads to systematic underestimation of venous pressure. In practice, however, it is difficult to use even relatively simple landmarks, and on attempts to locate an external reference point to determine the CVP, measurements obtained by critical care nurses vary by several centimeters. Venous pulsations above the clavicle with the patient in the sitting position are clearly abnormal, because the distance from the right atrium is at least 10 cm. Estimated CVP correlates only modestly with direct measurement. Measurements made at the bedside, in units of centimeters of blood or water, require conversion to millimeters of mercury (1.36 cm H2O = 1.0 mm Hg), for comparison with values measured with catheterization.
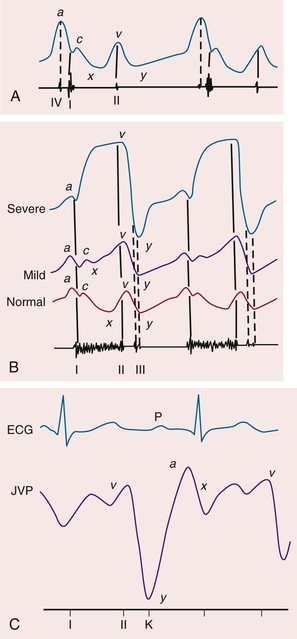
The venous waveforms include several distinct peaks: a, c, and v (see Fig. 11-2). The a wave reflects right atrial presystolic contraction, occurs just after the electrocardiographic P wave, and precedes the first heart sound (S1). Patients with reduced right ventricular (RV) compliance from any cause can have a prominent a wave. A cannon a wave occurs with atrioventricular (AV) dissociation and right atrial contraction against a closed tricuspid valve (Fig. 11-3). The presence of cannon a waves in a patient with wide complex tachycardia identifies the rhythm as ventricular in origin. The a wave is absent with atrial fibrillation (AF). The x descent reflects the fall in right atrial pressure after the a wave peak. The c wave interrupts this descent as ventricular systole pushes the closed valve into the right atrium. In the neck, the carotid pulse also may contribute to the c wave. As depicted in Figure 11-3, the x¢ descent follows because of atrial diastolic suction created by ventricular systole pulling the tricuspid valve downward. In normal persons, the x¢ descent is the predominant waveform in the jugular venous pulse. The v wave represents atrial filling, occurs at the end of ventricular systole, and follows just after S2. Its height is determined by right atrial compliance and by the volume of blood returning to the right atrium from any source. The v wave is smaller than the a wave because of the normally compliant right atrium. In patients with ASD, the a and v waves may be of equal height; in TR, the v wave is accentuated (Video 11-1![]() ). With TR, the v wave will merge with the c wave because retrograde valve flow and antegrade right atrial filling occur simultaneously (see Fig. 11-3). The y descent follows the v wave peak and reflects the fall in right atrial pressure after tricuspid valve opening. Resistance to ventricular filling in early diastole blunts the y descent, as is the case with pericardial tamponade or tricuspid stenosis. The y descent will be steep when ventricular diastolic filling occurs early and rapidly, as with pericardial constriction or isolated, severe TR. The normal venous pressure should fall by at least 3 mm Hg with inspiration. A rise in venous pressure (or its failure to decrease) with inspiration (Kussmaul sign) is associated with constrictive pericarditis, and also with restrictive cardiomyopathy, pulmonary embolism, RV infarction, and advanced systolic heart failure. A Kussmaul sign (Video 11-2
). With TR, the v wave will merge with the c wave because retrograde valve flow and antegrade right atrial filling occur simultaneously (see Fig. 11-3). The y descent follows the v wave peak and reflects the fall in right atrial pressure after tricuspid valve opening. Resistance to ventricular filling in early diastole blunts the y descent, as is the case with pericardial tamponade or tricuspid stenosis. The y descent will be steep when ventricular diastolic filling occurs early and rapidly, as with pericardial constriction or isolated, severe TR. The normal venous pressure should fall by at least 3 mm Hg with inspiration. A rise in venous pressure (or its failure to decrease) with inspiration (Kussmaul sign) is associated with constrictive pericarditis, and also with restrictive cardiomyopathy, pulmonary embolism, RV infarction, and advanced systolic heart failure. A Kussmaul sign (Video 11-2![]() ) is seen with right-sided volume overload and reduced RV compliance. Normally, the inspiratory increase in right-sided venous return is accommodated by increased RV ejection, facilitated by an increase in the capacitance of the pulmonary vascular bed. In states of RV diastolic dysfunction and volume overload, the right ventricle cannot accommodate the enhanced volume, and the pressure rises.
) is seen with right-sided volume overload and reduced RV compliance. Normally, the inspiratory increase in right-sided venous return is accommodated by increased RV ejection, facilitated by an increase in the capacitance of the pulmonary vascular bed. In states of RV diastolic dysfunction and volume overload, the right ventricle cannot accommodate the enhanced volume, and the pressure rises.
The abdominojugular reflex or passive leg elevation can elicit venous hypertension. The abdominojugular reflex requires firm and consistent pressure over the upper abdomen, preferably the right upper quadrant, for at least 10 seconds. A sustained rise of more than 3 cm in the venous pressure for at least 15 seconds after resumption of spontaneous respiration is a positive response. The patient should be coached to refrain from holding the breath or performing a Valsalva-like maneuver, which can falsely elevate the venous pressure. The abdominojugular reflex can predict heart failure and a pulmonary artery wedge pressure higher than 15 mm Hg.19
Measuring the Blood Pressure
Auscultatory measurement of blood pressure (see also Chapter 43) yields lower systolic and higher diastolic values than direct intra-arterial recording.20 Nurse-recorded blood pressure usually is closer to the patient’s average daytime blood pressure. Blood pressure should be measured with the patient in the seated position, with the arm at the level of the heart, using an appropriate-size cuff (Table 11-3). The use of an inappropriately small cuff can result in overestimation of the true blood pressure, an issue of particular relevance in obese patients.
TABLE 11-3
Important Aspects of Blood Pressure Measurement

On occasion, the Korotkoff sounds may disappear soon after the first sound, only to recur later before finally disappearing as phase 5. This auscultatory gap is more likely to occur in older, hypertensive patients with target organ damage. The systolic pressure should be recorded at the first Korotkoff sound and not when the sound reappears. This finding should be distinguished from pulsus paradoxus (see later). Korotkoff sounds may be heard all the way down to 0 mm Hg with the cuff completely deflated in children, in pregnant patients, or in patients with chronic severe AR, or in the presence of a large arteriovenous fistula. In these cases, both the phase 4 and phase 5 pressures should be noted.
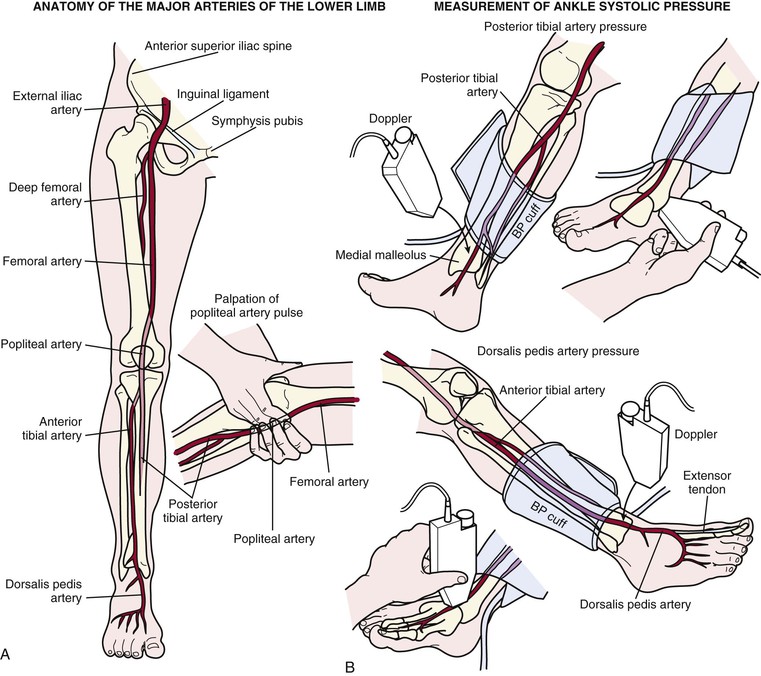
Blood pressure should be measured in both arms either in rapid succession or simultaneously; normally the measurements should differ by less than 10 mm Hg, independent of handedness. As many as 20% of normal subjects, however, exhibit a left-right arm blood pressure differential of more than 10 mm Hg in the absence of symptoms or other examination findings. A blood pressure differential of more than 10 mm Hg can be associated with subclavian artery disease, supravalvular aortic stenosis, aortic coarctation, or aortic dissection. Systolic leg pressures may exceed arm pressures by as much as 20 mm Hg; greater leg-arm systolic blood pressure differences are seen in patients with severe AR (Hill sign) and patients with extensive and calcified lower extremity peripheral arterial disease (PAD). Leg blood pressure should be measured using large thigh cuffs with auscultation at the popliteal artery or using a standard large arm cuff on the calf with simultaneous auscultation or palpation at the posterior tibial artery (Fig. 11-4). Measurement of lower extremity blood pressures constitutes the basis of the ankle-brachial index (ABI) (see Chapter 58).
Consideration should be given to ambulatory blood pressure monitoring when uncertainty exists about the significance of recordings obtained in the clinic. This approach is especially useful for the patient with suspected “white coat hypertension” (see Chapter 43).21 Measurement of normal or even low blood pressures in the face of evidence of hypertensive end organ damage should suggest masked hypertension caused by severe PAD.
Orthostatic hypotension (a fall in blood pressure of more than 20 mm Hg systolic and/or more than 10 mm Hg diastolic in response to moving from the supine to the standing position within 3 minutes) may be accompanied by a lack of compensatory tachycardia, a response suggestive of autonomic insufficiency, as can occur in patients with diabetes or Parkinson disease. The heart rate–blood pressure response to standing also depends on age, hydration, medications, food, conditioning, and ambient temperature and humidity.
An increase in pulse pressure can represent increased vascular stiffness, usually secondary to aging or atherosclerosis. Aortic stiffness is increased in patients with Marfan syndrome and other connective tissue disorders and may contribute to risk for dissection. Peripheral indices may not correlate well with central aortic stiffness, which is a primary determinant of ventricular-vascular coupling. One measure, the augmentation index, is the percentage increase in systolic pressure created by the premature return of the reflected wave during late systole.
Assessing the Pulses
The carotid artery pulse wave occurs within 40 milliseconds of the ascending aortic pulse and reflects aortic valve and ascending aortic function. The temporal arteries can be easily palpated to aid in the diagnosis of temporal arteritis. One of the two pedal pulses may not be palpable in a normal subject as a consequence of unusual anatomy (posterior tibial, less than 5%; dorsal pedis, less than 10%), but each pair should be symmetric. True congenital absence of a pulse is rare, and in most cases, pulses can be detected with a handheld Doppler device when not palpable. Simultaneous palpation of the brachial or radial pulse with the femoral pulse should be performed in patients with hypertension to screen for aortic coarctation.
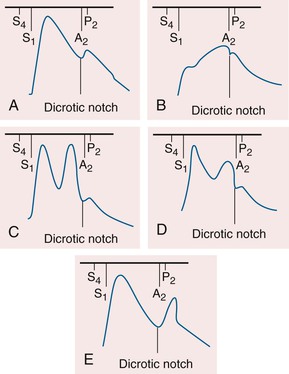
The contour of the pulses depends on the stroke volume, ejection velocity, vascular capacity and compliance, and systemic resistance. The palpable pulse reflects the merging of the antegrade pulsatile flow of blood and reflection of the propagated pulse returning from the periphery. The amplitude of the arterial pulse increases with distance from the heart. Normally, the incident (percussion) wave begins with systolic ejection (just after S1) and is the predominant monophasic pulse appreciated at the bedside (Fig. 11-5). The incisura or dicrotic notch identifies aortic valve closure. A bounding pulse may occur in hyperkinetic states such as fever, anemia, and thyrotoxicosis, or in pathologic states such as severe bradycardia, AR, or arteriovenous fistula. A bifid pulse is created by two distinct pressure peaks. This phenomenon may occur with fever or after exercise in a normal person and is consistent with increased vascular compliance. With chronic severe AR, a large stroke volume ejected rapidly into a noncompliant arterial tree produces a reflected wave of sufficient amplitude to be palpated during systole, rendering the pulse bifid. Hypertrophic obstructive cardiomyopathy (HOCM) can rarely produce a bifid systolic pulse with percussion and tidal waves (see Fig. 11-5).
A fall in systolic pressure of more than 10 mm Hg with inspiration (pulsus paradoxus) is considered pathologic and a sign of pericardial or pulmonary disease; this phenomenon also can occur in obesity22 and pregnancy without clinical disease. Pulsus paradoxus is detected by noting the difference between the systolic pressure at which the Korotkoff sounds are first heard (during expiration) and the systolic pressure at which the Korotkoff sounds are heard with each beat, independent of respiratory phase. Between these two pressures, the sounds will be heard only intermittently (during expiration). Appreciation of this finding requires a slow release of the cuff pressure. Conditions such as tachycardia, AF, and tachypnea make its assessment difficult. Pulsus paradoxus may be palpable when the pressure difference exceeds 15 to 20 mm Hg (see Chapter 71). Pulsus paradoxus is not specific for pericardial tamponade and can accompany massive pulmonary embolus, hemorrhagic shock, severe obstructive lung disease, or tension pneumothorax.
Pulsus alternans is defined by the beat-to-beat variability of the pulse amplitude (Fig. 11-6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree