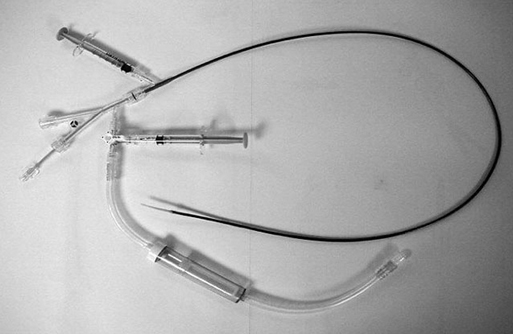
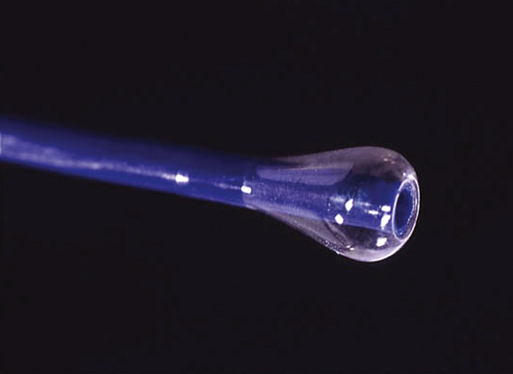
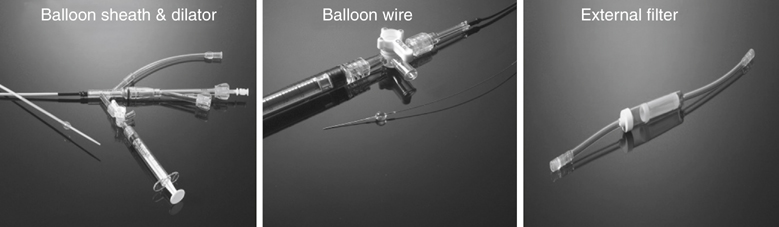
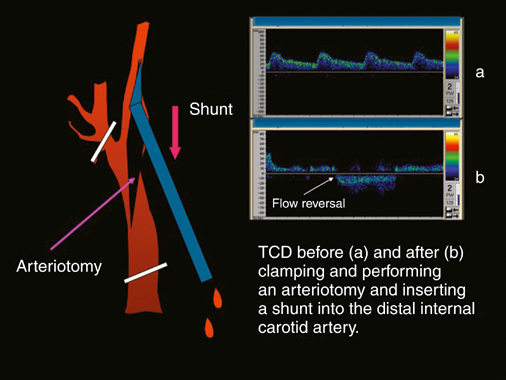
Years ago one of us (JCP) observed, during an open carotid endarterectomy using transcranial Doppler monitoring, that clamping the common carotid artery (CCA) and external carotid artery (ECA) and inserting a shunt in the distal end of the arteriotomy induced flow reversal in the middle carotid artery (MCA) if the other end of the shunt was left open to the air (Figure 1). Initially we were using the same principle through a small incision made at the base of the neck. We used a short 7-Fr introducer sheath placed in the CCA facing the lesion and occluding the ECA with a coronary balloon. The side port of the arterial line was then connected to a side port of a sheath placed in the internal jugular vein. Very soon we developed a long arterial sheath for femoral access with an occlusion balloon at its distal end (Figure 2). We designed the tip of the catheter in such a way that particles could not be trapped around the distal guiding catheter (Figure 3). We also developed an ECA occlusion balloon mounted in a hypotube and an external blood filter to be placed in between the side port of the arterial sheath and a venous sheath placed in the femoral vein. This early flow reversal device became known as the Parodi AntiEmboli System (PAES) (ArteriA Medical Science, San Francisco, CA) (Figure 4). The PAES is a closed system that allows arrest of ICA flow, continuous passive ICA flow reversal, or augmented active ICA flow reversal so that any particles released during CAS pass retrograde through the catheter and are retrieved in the arteriovenous conduit filter outside the body.
Reversal of Cerebral Blood Flow to Prevent Stroke during Percutaneous Carotid Artery Angioplasty and Stenting
Principles of Flow Reversal
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree