Retrograde SMA Angioplasty and Stenting in Patients with Acute Intestinal Ischemia
Mark C. Wyers
Mortality related to acute mesenteric arterial occlusion remains very high. Historically, acute superior mesenteric artery (SMA) occlusion from an embolus may account for 40% to 50% of cases of acute mesenteric ischemia (AMI) and carries a greater than 50% risk of death. These patients can be treated with straightforward embolectomy. In contrast, terminal thrombotic occlusion accounts for approximately 20% to 35% of cases of AMI and has a mortality of nearly 80% for several reasons. There may be a longer delay in diagnosis because of pre-existing chronic symptoms, coexistent malnutrition, multiple vessel involvement, and larger areas of bowel necrosis. Patient survival is dependent on prompt recognition and revascularization before ischemia progresses to intestinal gangrene.
Surgical revascularization is complex because mesenteric bypass is time consuming and can be limited by diseased inflow vessels and peritoneal soilage. Retrograde open mesenteric stenting (ROMS) is a hybrid endovascular–surgical treatment that was first described by Milner et al. and has been further modified and championed by Wyers et al. For these patients with emergent need for exploration, this hybrid open/interventional approach for treatment of acute atherosclerotic SMA thrombosis is an efficient, less-invasive mesenteric revascularization while not compromising important general surgical principles. Since these initial reports, others have reported similar success with this technique and suggested a lower mortality with ROMS compared to the historical mortality associated with emergent mesenteric bypass. ROMS has application to the subset of AMI patients that present with acute on chronic, or thrombocclusive mesenteric ischemia. It is indicated when there is an emergent need for mesenteric revascularization together with a careful evaluation of the intestines. It may have several advantages over traditional mesenteric bypass:
It avoids problems with inflow vessels such as excessive calcification or occlusion of the iliac or infrarenal aorta;
It eliminates the need for any aortic clamping and minimizes the physiologic insult in this group of very ill patients;
It eliminates the potential of a prosthetic graft infection from gross peritoneal contamination;
When contamination is present it eliminates the time required for saphenous vein harvest and the difficulty of routing a vein graft without kinking;
It has a high rate of technical success even in cases where there has been a previous unsuccessful attempt at antegrade percutaneous endovascular recanalization.
There are no absolute contraindications for the ROMS procedure. Appropriate imaging and endovascular equipment are fairly basic. A hybrid endovascular suite is optimal but a mobile C-arm works quite well.
It is important to point out that a previous failed endovascular approach is not a contraindication to an attempt at the retrograde approach. The rate-limiting step, as with all stenting procedures, is the ability to cross the lesion with a guidewire. Technical success is high presumably because of the superior pushability with sheath access so close to the obstruction. We noted in our original experience that technical success was 100%, even in 5 of the 6 patients reviewed who had previous unsuccessful attempts to cross the SMA from a percutaneous antegrade approach. A Dutch group recently also confirmed a high success rate with ROMS, 93% overall, including 4 of 4 who had had a preceding failed percutaneous attempt.
CT Angiography
It is worth highlighting the importance of a properly performed CT angiogram in the diagnosis of AMI and preoperative planning. The sensitivity and specificity of CTA approaches 95% for the diagnosis of AMI but relies heavily on accurate technique, as well as a complete evaluation of the scan’s vascular and nonvascular findings to arrive at an overall impression. Axial images and automated 3D reconstructions allow rapid interpretation of the vascular anatomy well out into third-order branches. Based on this, when thrombotic occlusion is seen, the endovascular team is alerted and the case is scheduled accordingly in the hybrid endovascular suite. The preoperative CT can also be used to select the correct stent size and to evaluate the SMA for calcification and to select the optimal site for retrograde access.
The CTA protocol should include an arterial and late arterial or early venous phase. The delay phase demonstrates the presence or absence of bowel wall enhancement. Fine, overlapping slices (0.5 to 1.5 mm collimation) should be made through the upper abdomen to minimize volume averaging and to evaluate the CA and SMA with sufficient detail. Traditional “positive” iodine-based oral contrast should not be given, as they tend to detract from image quality because of the artifact created by pooling of high-density material in the GI tract. Instead, a “negative” oral contrast, for example, water, can be given for enhanced image quality.
Resuscitation
Fluid resuscitation of the patient with AMI should begin immediately with isotonic crystalloid solution and continue with blood, if necessary. Electrolyte imbalances (hyperkalemia) should be monitored and corrected. Invasive monitoring (hourly urine output, continuous central pressure, and arterial pressure monitoring) is advisable from the beginning to ensure that all parameters are optimized prior to intravenous contrast administration and revascularization. Broad-spectrum antibiotics should be given to guard against translocated bacteria and sepsis. If there are no contraindications, intravenous heparin should also be administered to maintain a partial thromoplastin time greater than twice normal.
Sepsis and organ dysfunction in these patients are common and for the most part, they are managed as they would be in other situations. Vasopressors, however, may worsen ischemia in marginally viable bowel and exacerbate visceral vasospasm. Before the initiation of any vasopressor, volume resuscitation must be confirmed by the presence of adequate right heart filling pressures. Because of the large and ongoing fluid sequestration in these patients, serial examination and bladder pressure monitoring to recognize the development of abdominal compartment syndrome need to be performed. When necessary, better vasopressor options include dobutamine, lower dose dopamine, and occasionally epinephrine. Pure α-adrenergic agents, even after successful revascularization, should be avoided if possible.
Surgical planning should be coordinated between the general and vascular surgery services as much as possible. If the diagnosis of AMI is strongly considered, then it is best made known so that appropriate nursing staff and resources can be mobilized for the endovascular components of the case. This optimally will be performed in a hybrid operating suite if one is available. Alternatively, a general operating room with floating table and mobile fluoroscopy unit will work.
Positioning
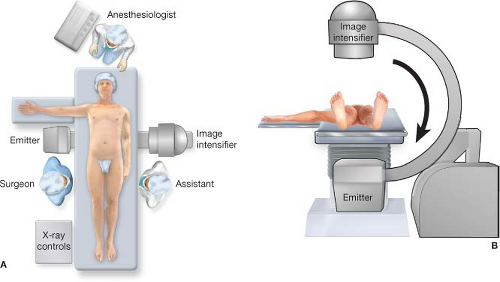
The patient is placed in the supine position with one arm, usually the left, tucked to allow the C-arm to move freely. The right arm can be left out to the side, but as high as possible and at a right angle so as not to interfere with the fluoroscopy unit when it is rotated for lateral projections. The entire abdomen and both proximal thighs are prepped to harvest a segment of greater saphenous vein to be used as a vein patch or for femoral arterial access if necessary (Fig. 5.1). Position of the table mounted retractor system is standard on the patient’s left but be aware of its potential to interfere with the C-arm. If there is inadequate clearance, the post for the retractor can be placed low on the bed near the left hip as an alternative. A nasogastric tube is placed to decompress the stomach. Standard fluoroscopy radiation safety practices are observed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree