Previous trials in elderly patients with ST-elevation myocardial infarction (STEMI) have not shown a definitive benefit of primary percutaneous coronary intervention (PPCI) by transfemoral approach over thrombolysis. The transradial approach (TRA) is associated with a significant decrease in mortality, MACE (Major Adverse Cardiac Event), and serious access site complications compared with the transfemoral approach. We have evaluated clinical outcomes in a cohort of real-life unselected ≥75-year-old patients with STEMI treated by TRA-PPCI. This is a single-center prospective, observational registry of consecutive patients with STEMI who underwent PPCI between February 2007 and February 2013. MACE was defined as death, reinfarction, or stroke. A total of 307 patients were treated by PPCI, 293 (95.1%) with TRA-PPCI (mean age 80 ± 2 years, 42% women). Patients had high co-morbidity levels (cardiogenic shock on admission 8.5%, previous myocardial infarction 11.6%, diabetes 30.4%, previous renal failure 25.6%, previous PCI 9.6%, and peripheral arterial disease 14.3%); IIbIIIa inhibitors were used in 45.1% of patients. Severe bleeding and need for transfusion were recorded for 6.5% and 9.9% of patients, respectively. In-hospital mortality, 1-year mortality, and 1-year MACE were 11.9%, 17.4%, and 22.2%, respectively. Excluding 25 patients with cardiogenic shock on admission, the in-hospital mortality, 1-year mortality, and 1-year MACE were 7.8%, 13.1%, and 17.9%, respectively. In conclusion, TRA-PPCI was feasible in the vast majority of elderly patients with STEMI. In-hospital mortality, 1-year mortality, and 1-year MACE were lower than reported for transfemoral access, suggesting a benefit of the TRA in these patients.
The Guidelines of the European Society of Cardiology recommend the transradial approach (TRA) in patients with STEMI if performed by an experienced radial operator (recommendation class IIa, level of evidence B). In these patients, TRA is associated with a significant decrease in mortality, MACE (Major Adverse Cardiac Event), and serious access site complications compared with the transfemoral approach. The present study analyzed outcomes of TRA-primary percutaneous coronary intervention (PPCI) in a real-life cohort of unselected ≥75-year-old patients with STEMI.
Methods
A prospective, single-center observational registry of all consecutive patients with STEMI treated with PPCI from February 2007 through February 2013 was used. The center serves an area of 817,000 inhabitants (Barcelonès Nord-Maresme, North of the Barcelona metropolitan area, Spain), and the organizational details for this local network have been published elsewhere.
PPCI was indicated in patients with chest pain and an electrocardiogram showing ST-segment elevation in 2 or more contiguous leads, with a minimum of 0.1 mV in frontal leads and 0.2 mV in precordial leads or with new or previously unknown left bundle branch block. Exclusion criteria included the presence of end-stage disease, severe co-morbidities, or a lack of arterial vascular access. Age was not a reason for exclusion. Data on patient demographics and procedure characteristics were collected prospectively.
The revascularization strategy during PPCI (access site, IIbIIIa inhibitors administration, and manual thrombectomy) was carried out according to the criteria of the interventionalist performing the procedure. All patients who received IIbIIIa inhibitors were treated with abciximab. “Cardiogenic shock” was defined as systolic blood pressure <90 mm Hg or the need for vasopressors to maintain blood pressure >90 mm Hg combined with signs of peripheral hypoperfusion (coldness and/or pallor in the extremities, oliguria, or a decrease in level of consciousness). “MACE” was defined as death, reinfarction, or stroke during hospitalization. “Severe bleeding” was defined as intracranial, respiratory, gastrointestinal, genitourinary, or access site–related bleeding that was life threatening or required blood transfusion. “Renal failure” was defined as an estimated glomerular filtration rate at admission <60 ml/min per 1.73 m 2 (using Cockcroft-Gault formula). “Acute renal failure” was defined as the impairment of renal function measured as either a 25% increase in serum creatinine from baseline or 0.5 mg/dl increase in absolute value. “Mechanical complication” was defined as free wall rupture resulting in cardiac tamponade, acute mitral valve regurgitation, or acute interventricular communication. “Stroke” was defined as neurologic deficit of cerebrovascular cause transient or persisting beyond 24 hours.
All patients provided informed consent allowing free access to the data used in this study. The study protocol was approved by the local ethics committee.
Categorical variables were described as n (%). Continuous variables were defined as mean ± SD and were compared using 1-way analysis of variance. Categorical variables were defined as n (%) and were compared using Pearson’s chi-square test. Variables that did not follow a normal distribution were defined as median (interquartile range) and were compared using the nonparametic Kruskal-Wallis test. Significance was defined as p <0.05 in bilateral contrast. Combined adverse events were evaluated on a per-patient hierarchical basis; thus, each patient could provide only 1 hard event per event type. The univariate analysis of MACE was performed using a binary logistic regression analysis. The multivariate analysis of MACE was performed with a binary logistic regression analysis including age, gender, and the variables that had a p <0.1 in the univariate analysis: diabetes, renal failure, anterior infarction, and cardiogenic shock. Data analysis was performed using the statistical program SPSS version 16.0 (SPSS Inc., Chicago, Illinois).
Results
Between February 2007 and February 2013, 1,516 consecutive patients with STEMI were treated by PPCI in our center: 307 (20.2%) were ≥75 years ( Figure 1 ). Of these patients, 14 (4.9%) with femoral or cubital access were excluded from the analysis. Radial access was used in 293 patients (95.1%) with a mean age of 80 ± 2 years (42% women). A total of 25 patients (8.5%) had cardiogenic shock on admission.
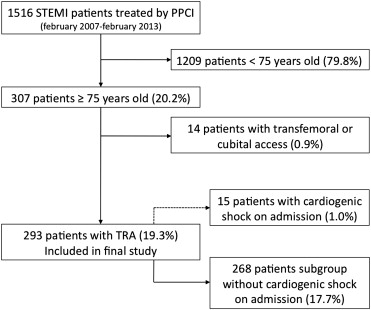
Table 1 provides the main baseline characteristics of the patients included in the study. Table 2 provides the main procedural characteristics. By intention to treat, radial access was from the right side in 94%. The incidence of crossover was 7.5% (29.4% to contralateral radial and 70.6% to femoral). Table 3 provides the main clinical outcomes on follow-up. In the 18 patients who had severe bleeding, the location was gastrointestinal in 8 patients (2.7%), genitourinary in 8 patients (2.7%), intracranial in 1 patient (0.3%), and pulmonary in 4 patients (1.4%). Table 4 provides the univariate analysis for in-hospital MACE and Table 5 provides the multivariate analysis for in-hospital MACE.
| Variable | All Patients (n = 293) | No Cardiogenic Shock (n = 268) | Cardiogenic Shock (n = 25) | p |
|---|---|---|---|---|
| Age (yrs) | 80 ± 4 | 80 ± 4 | 80 ± 4 | 0.88 |
| Women | 123 (42.0%) | 117 (43.7%) | 6 (24.0%) | 0.06 |
| Hypertension | 218 (74.4%) | 203 (75.7%) | 15 (60.0%) | 0.09 |
| Diabetes mellitus | 89 (30.4%) | 78 (29.1%) | 11 (44.0%) | 0.17 |
| Diabetes on insulin | 18 (6.1%) | 15 (5.6%) | 3 (12.0%) | 0.19 |
| Dyslipidemia | 134 (45.7%) | 123 (45.9%) | 11 (44.0%) | 0.99 |
| Current smoker | 23 (7.8%) | 20 (7.5%) | 3 (12.0%) | 0.43 |
| Peripheral arterial disease | 42 (14.3%) | 36 (13.4%) | 6 (24.0%) | 0.14 |
| Previous myocardial infarction | 34 (11.6%) | 30 (11.2%) | 4 (16.0%) | 0.51 |
| Previous percutaneous coronary intervention | 28 (9.6%) | 24 (9.0%) | 4 (16.0%) | 0.28 |
| Previous coronary bypass | 1 (0.3%) | 1 (0.4%) | 0 (0%) | — |
| Previous renal failure | 75 (25.6%) | 61 (22.8%) | 14 (56.0%) | 0.001 |
| Previous hemodialysis | 4 (1.4%) | 4 (1.5%) | 0 (0%) | — |
| Variable | All Patients (n = 293) | No Cardiogenic Shock (n = 268) | Cardiogenic Shock (n = 25) | p |
|---|---|---|---|---|
| STEMI location | 0.96 | |||
| Anterior | 140 (47.8%) | 127 (47.4%) | 13 (52%) | |
| Inferior (posterior) | 138 (47.1%) | 127 (47.4%) | 11 (44%) | |
| Lateral | 14 (4.8%) | 13 (4.9%) | 1 (4%) | |
| Killip class on admission | — | |||
| I | 198 (67.6%) | 198 (73.9%) | — | |
| II | 50 (17.1%) | 50 (18.7%) | — | |
| III | 19 (6.5%) | 19 (7.1%) | — | |
| IV | 25 (8.5) | — | 25 (100%) | |
| FMC-to-device time <120 min | 168 (57.7%) | 157 (58.6%) | 12 (48%) | 0.21 |
| FMC-to-device time, median (interquartile range) (min) | 113 (84–148) | 113 (83–148) | 125 (91–152) | 0.96 |
| Crossover | 22 (7.5%) | 19 (7.1%) | 3 (12%) | 0.29 |
| IIbIIIa inhibitors administration | 132 (45.1%) | 120 (44.8%) | 12 (48%) | 0.46 |
| Manual thrombectomy | 208 (71.0%) | 188 (70.1%) | 20 (80%) | 0.21 |
| Intra-aortic balloon pumping use | 8 (2.7%) | 2 (0.7%) | 6 (24%) | <0.0001 |
| Final TIMI 3 flow | 261 (89.1%) | 244 (91%) | 17 (68%) | 0.003 |
| LVEF (%) | 46 ± 13 | 48 ± 12 | 34 ± 16 | <0.0001 |
| Target coronary artery | 0.04 | |||
| Left mail | 9 (2.6%) | 6 (2.2%) | 3 (12%) | |
| Left anterior descendant | 158 (45.8%) | 128 (47.8%) | 9 (36%) | |
| Left circumflex | 40 (11.6%) | 29 (10.8%) | 4 (16%) | |
| Right coronary artery | 139 (40.2%) | 105 (39.2%) | 9 (36%) | |
| Number of vessels diseased | 1.9 ± 0.8 | 1.9 ± 0.8 | 2.1 ± 0.8 | 0.25 |
| Number of vessels treated | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.4 | 0.006 |
| Number of lesions treated | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.5 ± 0.6 | 0.05 |
| Number of stents implanted | 1.3 ± 0.6 | 1.3 ± 0.6 | 1.6 ± 0.9 | 0.06 |
| Contrast dose (ml) | 195 ± 93 | 190 ± 90 | 243 ± 113 | 0.007 |
| Days of hospitalization | 7.5 ± 8.7 | 7.1 ± 7.5 | 16.5 ± 3.3 | 0.009 |
| Variable | All Patients (n = 293) (%) | No Cardiogenic Shock (n = 268) (%) | Cardiogenic Shock (n = 25) (%) | p |
|---|---|---|---|---|
| In-hospital mortality | 26 (12.3) | 21 (7.8) | 15 (60) | <0.0001 |
| In-hospital MACE | 38 (13.0) | 26 (9.7) | 16 (64) | <0.0001 |
| Mechanical complication | 6 (2.0) | 4 (1.5) | 2 (8) | 0.08 |
| In-hospital stroke | 3 (1.0) | 2 (0.7) | 1 (4) | 0.23 |
| Acute renal failure | 48 (16.4) | 37 (13.8) | 11 (44) | 0.001 |
| In-hospital severe bleeding | 19 (6.5) | 14 (5.2) | 5 (20) | 0.01 |
| 1-yr mortality | 51 (17.4) | 35 (13.1) | 16 (64) | <0.0001 |
| Cardiac | 39 (13.3) | 25 (9.4) | 14 (56) | |
| Noncardiac | 10 (3.4) | 8 (3.0) | 2 (8) | |
| Unknown | 1 (0.3) | 1 (0.4) | 0 (0) | |
| 1-yr myocardial infarction | 12 (4.1) | 10 (3.7) | 2 (8) | 0.27 |
| 1-yr stent thrombosis | 8 (2.7) | 6 (2.2) | 2 (8) | 0.14 |
| 1-yr target vessel revascularization | 13 (4.4) | 11 (4.1) | 2 (8) | 0.31 |
| 1-yr nontarget vessel revascularization | 30 (10.2) | 30 (11.2) | 0 (0) | 0.90 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


