Essentials of Diagnosis
- Predominant biventricular diastolic dysfunction with lesser impairment of systolic performance.
- Symptomatic presentation:
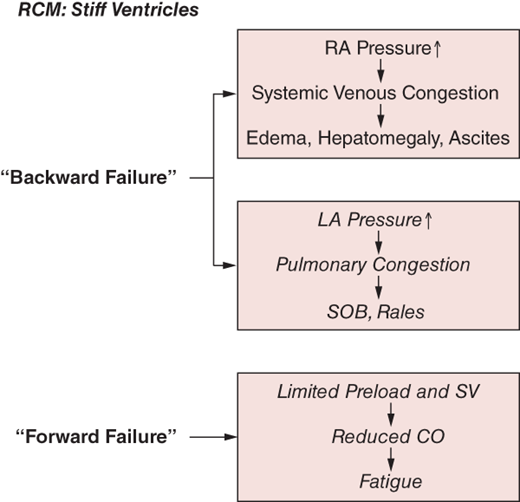
- Chronic biventricular “backward failure” manifest as dyspnea and peripheral edema (often hepatomegaly and ascites).
- Reduced preload limits cardiac output (fatigue).
- Chronic biventricular “backward failure” manifest as dyspnea and peripheral edema (often hepatomegaly and ascites).
- Echocardiography: small stiff ventricles, preserved systolic function (until late stages), dilated atria and diastolic dysfunction by Doppler.
- Many disorders manifest restrictive “physiology” and must be excluded (eg, hypertrophic cardiomyopathy and constrictive pericarditis).
- Cardiac magnetic resonance imaging powerful: delineates myocardial infiltration, inflammation, and fibrosis and assesses pericardium, thereby helping establish underlying disorder.
- Tissue biopsy may be necessary for definitive diagnosis in some disorders (eg, amyloidosis).
Overview
Restrictive cardiomyopathies (RCM) are indolent disabling diseases resulting from pathophysiologic processes that induce predominant diastolic chamber dysfunction with lesser impairment of systolic performance. RCM is characterized by small stiff ventricles with progressive impairment of diastolic filling, leading to the hemodynamic conundrum of low preload but high filling pressures (Figure 24–1). This pattern of diastolic dysfunction leads to dilated atria and elevated mean atrial pressures, resulting clinically in biventricular “backward failure” manifest as pulmonary venous congestion (dyspnea) as well as systemic venous pressure elevation (peripheral edema). Systolic function is preserved in most cases, depending on the underlying cause (at least in the presenting stages of most of the underlying diseases). However, despite intact systolic function, the restrictive constraints on true ventricular preload limit stroke volume, thereby resulting in low cardiac output (fatigue) and ultimately hypoperfusion.
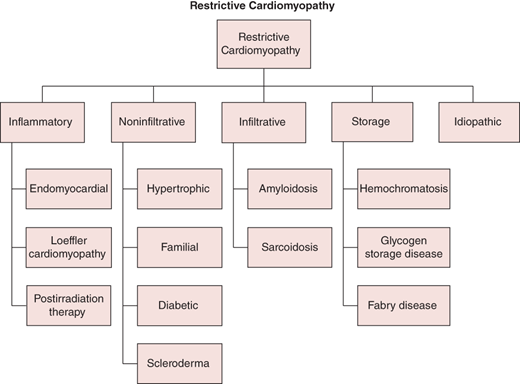
The abnormal diastolic properties of the ventricle are typically attributable to abnormalities of the myocardium (infiltration, inflammation, or fibrosis) or the endomyocardial surface (inflammation and scarring). The RCM classification (Figure 24–2) may be most easily considered based on underlying pathophysiologic process as infiltrative (eg, amyloidosis), storage (eg, hemochromatosis), inflammatory (eg, hypereosinophilic syndrome), noninfiltrative (eg, diabetic), or idiopathic. Identification of specific infiltrative processes may have prognostic and therapeutic implications. Of note, secondary restrictive physiology can develop at a late stage in hypertrophic, dilated, valvular, hypertensive, and ischemic heart disease.
Pertinent Aspects of Normal Hemodynamics
An appreciation of the physiology of the venous circulations and the dynamic effects of intrathoracic pressure (ITP) and respiratory motion on cardiovascular physiology is critical to understanding the hemodynamic pathophysiology of RCM and differentiating it from other conditions, particularly constrictive pericarditis (CP). Under physiologic conditions, venous return to both atria is biphasic, with a systolic peak determined by atrial relaxation (“X” descent of the atrial and jugular venous pressure [JVP] waveforms) and a diastolic peak determined by tricuspid valve (TV) resistance and right ventricular (RV) compliance (“Y” descent). Respiratory oscillations exert complex effects on cardiac filling and dynamics, with disparate effects on the right and left heart attributable to differences in their anatomic relationships to the respective venous return systems relative to the intrapleural space. Normally, inspiration induces decrements in ITP (–25 to –30 mm Hg), which are transmitted through the pericardium to the cardiac chambers. On the right heart, these decrements in ITP enhance the filling gradient from the extrathoracic systemic veins, thereby augmenting venous return and increasing right heart filling and output. In contrast, the left heart and its tributary pulmonary veins are entirely intrathoracic. Therefore, inspiratory decrements in ITP are evenly distributed, and thus, mitral valve (MV) flow and left ventricular (LV) filling are essentially unchanged throughout the respiratory cycle.
Pathophysiology
The pathognomonic feature of RCM is stiffness of the ventricular walls, which impairs diastolic filling, resulting in limited ventricular preload (and therefore stroke volume) despite increased diastolic filling pressures. Echocardiography (echo) documents small ventricles, often with thick walls. By invasive hemodynamic assessment, RCM is characterized by elevated ventricular diastolic pressures (Figures 24–3 and 24–4), often with a mild diastolic “dip and plateau” or “square root” pattern, a nonspecific indicator of stiff noncompliant chambers, a phenomenon also seen in other conditions such as CP (discussed later). Echo-Doppler demonstrates classic features of impaired diastolic function (impaired myocardial relaxation with increased early LV filling velocity, decreased atrial filling velocity, and decreased isovolumic relaxation time). Ventricular systolic function is typically preserved until the later stages of the underlying disease. Biatrial enlargement reflects ventricular noncompliance and may also result from primary atrial myocardial involvement by the disease process (eg, amyloidosis). Atrial filling pressures are elevated, and the X and Y descents tend to be relatively blunted; in cases in which the atria are primarily involved by the restrictive process, the A wave may be depressed (see Figure 24–3). In RCM, a pattern of “elevated and equalized” chamber diastolic filling pressures indicating pancardiac “stiffness” is common, but this nonspecific pattern is also seen in other diseases, particularly pericardial constraint conditions (eg, CP). However, in RCM, left-sided pressures are typically somewhat higher than right due to the greater intrinsic stiffness of the LV. This difference may be amplified by maneuvers that augment ventricular filling such as volume infusion, leg-raising, or post–premature ventricular contraction increased filling time. Disproportionate left heart stiffness may also result in moderate pulmonary hypertension (a subtle distinguishing feature from CP).
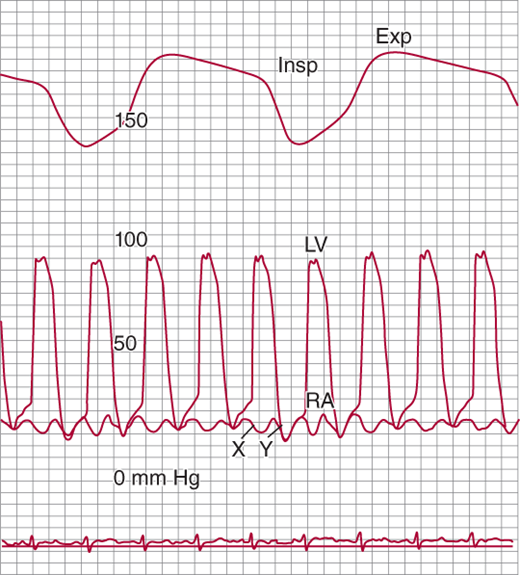
Figure 24–3.
Simultaneous recordings of left ventricular (LV) and right atrial (RA) pressures. Note the marked “W” or “M” pattern in the RA pressure tracing with prominent X and Y descents and with no fall with inspiration (Kussmaul sign). The nasal respirometer tracing is also shown. Exp, expiration; Insp, inspiration. (From Higano ST, et al. Cath Cardiovasc Inter. 1999;46: 473–86.)
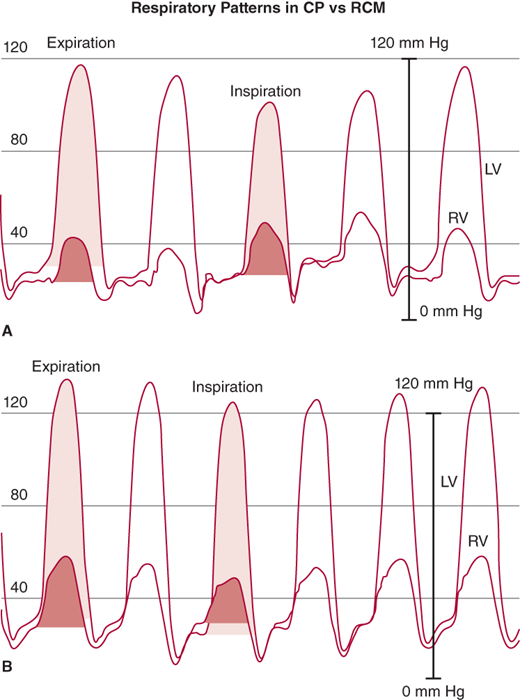
Figure 24–4.
Left ventricle (LV) and right ventricle (RV) high-fidelity manometer pressure traces from two patients during expiration and inspiration. Note that both patients have early rapid filling and elevation and end-equalization of the LV and RV pressures at end expiration. A: A patient with surgically documented constrictive pericarditis. During inspiration, there is an increase in the area of the RV pressure curve (orange-shaded area) compared with expiration. The area of the LV pressure curve (yellow-shaded area) decreases during inspiration as compared with expiration. B: A patient with restrictive myocardial disease documented by endomyocardial biopsy. During inspiration, there is a decrease in the area of the RV pressure curve (orange-shaded area) as compared with expiration. The area of the LV pressure curve (yellow-shaded area) is unchanged during inspiration as compared with expiration. (From Talreja DR, et al. J Am Coll Cardiol. 2008;51:315.)
In RCM, inspiratory changes in ITP are fully transmitted through the pericardium to the cardiac chambers. However, chamber noncompliance contributes to a relative lack of respiratory variation in cardiac filling, and therefore, mitral and tricuspid flow velocity during respiration are typically decreased. Because of the lack of pericardial constraint and a relatively noncompliant interventricular septum, there is minimal ventricular interdependence, and thus inspiratory effects on ventricular systolic pressures are concordant (ie, there is little change in peak ventricular systolic pressures with respiration and they move in the same direction) (see Figure 24–4); as will be discussed, the pressure pattern in CP is “discordant.” As RCM progresses and right-sided chambers become less distensible, the respiratory swings in pressure diminish further. At its most severe, noncompliance to inflow results in the “Kussmaul sign,” an inspiratory increase in venous return that cannot be accommodated by the stiff right heart, resulting in inspiratory elevation of JVP (see Figure 24–3). Doppler flow patterns show blunting of the expected physiologic respiratory increases in flow across the TV and minimal change across the MV.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree