T
Primary Tumor
T1
Tumor ⩽3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than a lobar bronchus (i.e., not in the main bronchus) T1a Tumor ⩽2 cm in greatest dimension T1b Tumor >2 cm but ⩽3 cm in greatest dimension
T2
Tumor >3 cm but ⩽7 cm or tumor with any of the following features: Involves main bronchus, ⩾2 cm distal to the carina Involves visceral pleura Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung
T2a
Tumor >3 cm but ⩽5 in greatest dimension T2b Tumor >5 cm but ⩽7 cm in greatest dimension
T3
Tumor >7 cm that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, or tumor in the main bronchus <2 cm distal to the carina but without involvement of the carina or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe
T4
Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina, separate tumor nodule(s) in a different, ipsilateral lobe
N
Regional Lymph Nodes
N0
No regional lymph node metastasis
N1
Metastasis to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes, and intrapulmonary nodes including involvement by direct extension
N2
Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s)
N3
Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s)
M
Distant Metastasis
M0
No distant metastasis
M1
Distant metastasis M1a Separate tumor nodule(s) in a contralateral lobe, tumor with pleural Nodule(s) or malignant pleural (or pericardial) effusion M1b Distant metastasis
Table 2.
Resultant stage groupings (TNM staging)
Stage grouping | TNM | |||
Stage IA | T1a,b | N0 | M0 | |
Stage IB | T2a | N0 | M0 | |
Stage IIA | T2b | N0 | M0 | |
T1a,b | N1 | M0 | ||
T2a | N1 | M0 | ||
Stage IIB | T2b | N1 | M0 | |
T3 | N0 | M0 | ||
Stage IIIA | T1–2a,b | N2 | M0 | |
T3 | N1–2 | M0 | ||
T4 | N0–1 | M0 | ||
Stage HIB | T4 | N2 | M0 | |
any T | N3 | M0 | ||
Stage IV | any T | any N | M1 |
The 7th revision of the TNM system for lung cancer staging was published in 2010 [4] (Table 1). Significant changes from the 6th edition aim to provide a stronger correlation between the TNM stage and survival data.
The T descriptor has been reclassified according to size of the primary tumor (Table 1):
T1 (<3 cm) is now split into T1a (<2 cm) and T1b (2–3 cm).
T2 is split into T2a (3–5 cm) and T2b (5–7 cm).
T3 includes tumors >7 cm. Additional pulmonary nodules are reclassified as either
T3, with location in the same lobe
T4, with nodules in another lobe on the same side
M1a, with nodules in the contralateral lung. The N descriptor remains unchanged [5] (Tables 1 and 3):
N1 disease refers to peribronchial and ipsilateral hilar metastases, including direct extension. All N1 nodes lie distal to the mediastinal pleural reflection and within the visceral pleura [5].
N2 disease refers to ipsilateral paratracheal and/or subcarinal lymph node metastases.
N3 disease refers to contralateral mediastinal, contralateral hilar, and ipsilateral or scalene or supraclavicular nodal metastases.
Table 3.
Lymph node map: Definitions of nodal stations
N2 nodes — all N2 nodes lie within the mediastinal pleural envelope |
1 Highest mediastinal nodes |
2 Upper paratracheal nodes |
3 Prevascular and retrotracheal nodes |
4 Lower paratracheal nodes |
5 Subaortic nodes (aorto-pulmonary window) |
6 Para-aortic nodes (ascending aorta or phrenic) |
7 Subcarinal nodes |
8 Paraesophageal nodes (below carina) |
9 Pulmonary ligament nodes |
N1 nodes — all N1 nodes lie distal to the mediastinal pleural reflection and within the visceral pleura |
10 Hilar nodes |
11 Interlobular nodes |
12 Lobar nodes |
13 Segmental nodes |
14 Sub segmental nodes |
Notably, for paratracheal lymph nodes, the left lateral border of the trachea and not the midline differentiates left from right. Thus, a pretracheal lymph node metastases may be N2 disease in lung cancer of the right lung, but N3 disease if the primary is located in the left lung (Fig. 1).
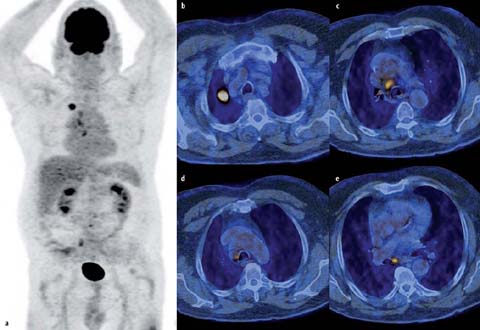
Fig 1 a–e.
FDG-PET/CT in a 76-year-old man. Images display an FDG-avid squamous cell carcinoma in the right upper lobe (a, b) as well as pretracheal (c), right paratracheal (d), and subcarinal (e) lymph node metastases. The classification pT1B pN2 cM0 was proven by histology. Notably, the left lateral border of the trachea differentiates left from right paratracheal lymph nodes. Thus, the pretracheal lymph node metastases (c) would not mean N2 but N3 disease if the lung tumor was in the left lung. Subcarinal lymph node metastases (e) are always considered N2 disease regardless of the location of the primary lung tumor
The M descriptor has been divided into M1a and M1b, for intrathoracic and distant spread respectively. Patients with malignant pleural effusions have been up-staged from T4 to M1 (Table 1).
The labeling of stages also was revised between the 6th and 7th editions (Table 2). For example, T2bN0 has moved from stage IB to stage IIA, and T2aN1from stage IIB to stage IIA. Patients with tumors >7 cm move from IB to IIB if there is no lymph node metastasis, and from IIB to IIIA if they have N1 lymph nodes. Patients without N3 lymph nodes but with an additional nodule in the same lobe have been moved from stage HIB to stage IIIA.
The new TNM staging system for lung cancer directly impacts treatment algorithms. Treatment approaches for NSCLC are being constantly adapted and may be shifted from standardized to personalized strategies. Formerly, stages I to IIIA were considered “operable,” while stages HIB and VI were considered “not to be operable”. This is no longer in line with current treatment guidelines. For instance, stage IIIA with N2 lymph node metastasis may be considered inoperable if lymph node metastases are bulky or in multiple lymph node levels that lie in the ipsilateral mediastinum. A curative operation may be considered if down-staging of N3 disease with neoadjuvant chemotherapy is successful. In stage IV disease, curative treatment approaches may be considered today even if oligometastatic disease is found with involvement of the adrenal glands or the brain. Last but not least, standardized treatment strategies are to be defined in adenocarcinomas with EGFR mutations or ALK rearrangements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


